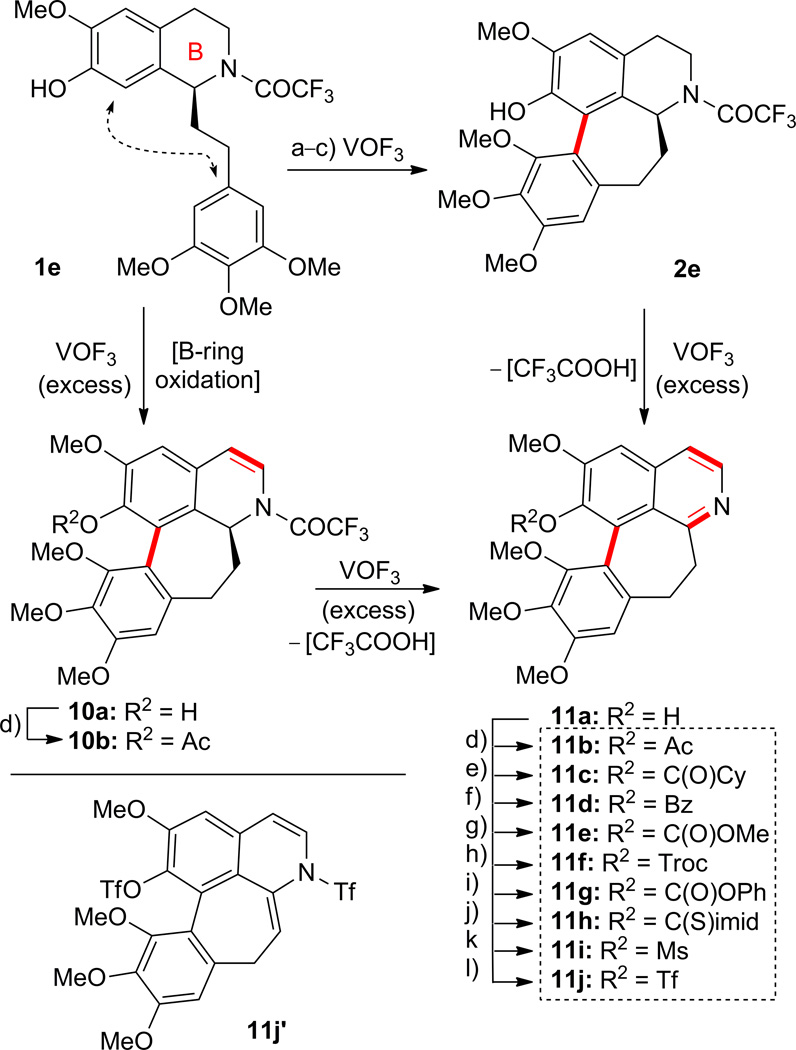
Scheme 3.
Reaction of compound 1e with vanadyl trifluoride (VOF3). Synthesis of compounds 2e, 10a,b, 11a–j,j′. Reagents and conditions: a) VOF3, TFA–TFAA–CH2Cl2 (10:1:100), 0 to 25 °C, 3 h, 1e → 10a (9%) plus 11a (17%); b) VOF3, TFA–TFAA–CH2Cl2 (75:1:0), 0 to 25 °C, 6 h, 1e → 2e (46%) plus 11a (27%); c) VOF3, TFA–TFAA–CH2Cl2 (10:1:150), 0 to 25 °C, 16 h, 1e → 11a (49%); d) AcCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11b (64%); e) CyC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11c (88%); f) PhC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11d (94%); g) MeOC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11e (54%); h) 2,2,2-trichloroethyl chloroformate, Et3N, CH2Cl2, 0 °C, 0.5 h, 11f (77%); i) PhOC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11g (56%); j) 1,1′-thiocarbonyldiimidazole, Et3N, CH2Cl2, 25 °C, 1 h, 11h (39%); k) MsCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11i (74%); l) Tf2O, Et3N, CH2Cl2, −78 °C, 0.5 h, 11j (37%) plus 11j′ (51%). TFA = 2,2,2-trifluoroacetic acid; TFAA = trifluoroacetic anhydride; Cy = cyclohexyl; Bz = benzoyl; Troc = 2,2,2-trichloroethoxycarbonyl; C(S)imid = thionoimidazolide.