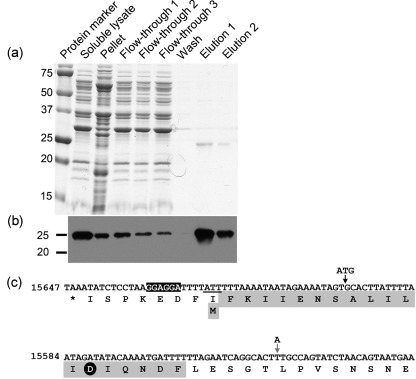
Fig. 2.
Purification and N-terminal sequence analysis of PncA–FLAG protein from B. burgdorferi. (a) Coomassie-stained SDS-PAGE gel of protein fractions collected during purification of the PncA–FLAG protein from B. burgdorferi. (b) Immunoblot of the protein fractions collected during purification of the PncA–FLAG protein from B. burgdorferi using antibodies against the FLAG-epitope tag. Numbers to the left of panels (a) and (b) indicate the masses in kDa of the protein standards. (c) Reverse complement of nt 15647–15525 from lp25. The corresponding translated single-letter amino acid sequence is shown below the nucleotide sequence. The sequence of the first 20 aa of native purified PncA–FLAG as determined by Edman degradation and N-terminal sequencing is highlighted in grey. The putative ribosome-binding site sequence is highlighted in black. The non-canonical translational start codon of native purified PncA–FLAG (ATT) is underlined. The aspartic acid residue conserved among the catalytic triad of PncA homologues is indicated by a black circle. The locations of the engineered ATG start codon and translational start site for construct pncA-FLAG A, and the nucleotide point mutation (T to A) to create an ATG start codon and translational start site for construct pncA-FLAG B (which reflects the current proposed translational start site for the pncA ORF), are indicated by vertical arrows (black and grey, respectively).