Abstract
Background
Oxidative stress and inflammation are associated with the development of inflammatory bowel diseases such as necrotizing enterocolitis. We tested the hypothesis that probiotics, prebiotics or synbiotics (a combination of pre- and probiotics) is effective for prevention of inflammatory responses to formula-feeding in the terminal ileum of neonatal rats.
Methods
Neonatal rats were exposed to hyperoxia/hypoxia during which they were either maternally-fed or hand-fed with formula supplemented with probiotics, prebiotics or synbiotics. A non-supplemented formula group served as controls. Cytokines and genes associated with oxidative stress and toll-like receptor signaling were determined in the terminal ileum. Data were compared to room air littermates.
Results
Exposure to hyperoxia/ hypoxia reduced growth accretion in maternally-fed pups. NEC-like symptoms and intestinal inflammatory markers were induced with formula feeding. Supplementation with probiotics, prebiotics or synbiotics decreased proinflammatory cytokines and downregulated genes involved in oxidative stress and toll-like receptor pathways, however, the effect was attenuated in hyperoxia/hypoxia.
Conclusions
The combination of suboptimal nutrition due to removal of breast milk and formula feeding with hyperoxia/hypoxia may increase susceptibility to oxidative stress, inflammation, and NEC. Probiotics, prebiotics and synbiotics are protective against oxidative stress and inflammation, but their efficacy may be reduced when administered during hyperoxia/hypoxia insults.
Keywords: Cytokines, hypoxia/hyperoxia, prebiotics, probiotics, synbiotics, toll-like receptors
Introduction
Necrotizing enterocolitis (NEC) is a devastating inflammatory condition of the gastrointestinal tract that afflicts approximately 12% of premature infants less than 32 weeks and less than 1500 grams [1]. Inflammation and tissue necrosis are the hallmarks of NEC. Oxidative stress and uncontrolled and/or exaggerated inflammatory responses in the immature gut secondary to abnormal intestinal bacterial colonization may play a central role in the pathogenesis of necrotizing enterocolitis. Oxidative stress and inflammation may contribute to the disruption of the protective gut barrier through various mechanisms; mitochondrial dysfunction resulting from inflammatory and oxidative injury may potentially be a significant source of apoptosis during NEC [2-4]. Emerging evidence suggests that the innate immune system, comprised of toll-like receptors (TLRs) and their associated molecules, plays a pivotal role in the response to invading pathogens and in the regulation and control of intestinal inflammation [5]. Epidermal growth factor (EGF), inflammatory mediators including tumor necrosis factor α (TNF-α) and interleukins like IL-1β and IL-6 also proposed to have significant roles in the pathogenesis of NEC [6,7].
Probiotics are “live microorganisms” (bacteria or yeast) which when administered in adequate amounts confer a health benefit on the host [8]. Prebiotics, fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS), are non-digestible food ingredients that benefit the host by stimulating growth and activity of beneficial bacteria. Synbiotics, a combination of probiotics and prebiotics that improves the survival of the probiotic organism and the intestinal environment, have also been shown to be beneficial [9-11]. It beneficially affects the host by improving the survival and implantation of live microbial dietary supplementation in the gastrointestinal tract, and thus improving host health and well being [12]. The use of probiotics has recently been shown to prevent NEC in extremely low birth weight (ELBW) infants [13]. However, the comparative effects of probiotics, prebiotics, and synbiotics on inflammatory cytokines, EGF, TLRs and oxidative stress in formula-induced bowel inflammation have not been studied.
Formula-fed rats produce various degrees of inflammatory changes and share many histopathological and clinical features of NEC seen in human preterm newborns [14]. Therefore, we used formula-fed neonatal rats to test our hypothesis that probiotics, prebiotics and/or synbiotics reduce inflammation and oxidative stress in the terminal ileum of formula-fed rats exposed to hyperoxia/hypoxia. Since ELBW infants frequently experience extremes of oxygen saturations, we performed our experiments during hyperoxia with brief hypoxia in order to be more clinically relevant.
Material and methods
This study was approved by the State University of New York, Downstate Medical Center Animal Care and Use Committee, Brooklyn, NY. Animals were cared for and handled according to the United States Department of Agriculture guidelines. Euthanasia was carried out according to the American Veterinary Medical Association Panel for Euthanasia guidelines.
Animals
Certified infection free timed pregnant Sprague Dawley rats (200-300 grams) carrying fetuses [6-17] of known gestational age (18 days) were purchased from Charles River Laboratories. The pregnant rats are placed in nesting cages and allowed to stabilize (from transportation) for 48 hours where they remained undisturbed until delivery of their pups. The animals were housed in with a 12 hours-day/12-hour night cycle and were provided standard laboratory diet and water ad libitum. Within 24 hours of birth, newborn pups delivering on the same day were pooled and randomly assigned to expanded litter of 18. Each pup were weighed and measured for linear growth (crown to rump length) every 24 hours.
Probiotic, prebiotic and synbiotic preparation
The rat pups were either maternally-fed or hand-fed with formula. The formula-fed groups were: 1) non-supplemented or formula only; 2) probiotics; 3) prebiotics; or 3) synbiotics. Similac Advance (Ross/Abbott) was used for formula only. Similac Advance formula was supplemented with live, freeze-dried yeast cells of the species Saccharomyces boulardii lyo (“Florastor Kids”, Biocodex, Inc. San Bruno, CA) and used in the probiotic group. Similac Advance Early Shield formula which contains FOS and GOS was used in the prebiotic group. Similac Advance Early Shield formula was supplemented with “Florastor Kids” and used in the symbiotic group.
Hyperoxia/Hypoxia
Newborn rat pups were exposed hyperoxia (50%) with brief hypoxia (12%) lasting 1-2 minutes every 6 hours from the first day of life (P0) to P3, in order to simulate frequent arterial oxygen desaturations frequently observed in ELBW preterm infants. On the day of birth, the hyperoxia/hypoxia litters were placed with the dams in specialized oxygen chambers (BioSpherix, New York) attached to an oxycycler (10”H x 22”W x 19”D, 55 pounds). Oxygen inside the chamber was continuously monitored using an oxygen analyzer (Ventronics, Temecula, CA), and recorded on a Dell Computer. Oxygen inside the chamber was controlled by the oxycycler which infuses nitrogen to reduce oxygen, or infuses oxygen to raise it. Carbon dioxide in the chamber was continuously monitored and removed. Temperature and humidity inside the chamber was continuously monitored and maintained at standard values. The 50%/12% oxygen atmosphere was achieved by mixing appropriate amounts of pure nitrogen and oxygen. The pups were kept at 50% O2 and the hypoxia profile was as follows: P0: 3 consecutive hypoxic episodes every 4 hours; P1: 3 consecutive hypoxic episodes every 5 hours; P3: 3 consecutive hypoxic episodes every 6 hours. Room air littermates remained in room air from P0-P3.
Sample collection
On P4, the surviving pups were killed by decapitation and samples from the terminal ileum were collected for histologic, biochemical, and molecular analyses. To identify the terminal ileum, the intestines were dissected out and biopsies were obtained from the most terminal area. For light microscopy, 2-3 biopsies (~1 cm) were obtained from four representative pups in each group. The specimens were placed in 10% neutral buffered formalin and taken to the pathology department for processing using graded alcohols and paraffin embedding. For analysis of growth factors, fresh tissue samples (200 mg) were rinsed in ice-cold PBS to remove blood elements and then placed in tubes containing ceramic beads and 1.0mL sterile normal saline. The samples were homogenized using a FastPrep instrument (MP Biomedicals, Solon, OH). The homogenates were centrifuged at 8000 rpm for 20 min at 4°C and filtered before assay for IL-1β, IL-6 and TNF-α. Levels in the samples were standardized using total cellular protein levels. For mRNA extraction, fresh tissue samples (100 mg) were rinsed in ice-cold PBS and placed nuclease-free microtubes and frozen immediately at -70°C until assay.
Tissue analysis
Cytokines in terminal ileum homogenates were analyzed using multiplex bead array (MILLIPLEX MAP Rat Cytokine/Chemokine Panel, Millipore). Gene expression of cytokines, toll-like receptors and oxidative stress were analyzed in the terminal ileum using real-time PCR arrays (SA Biosciences). Histology was performed using standard laboratory techniques.
Assay of EGF
EGF levels in the terminal ileum homogenates were measured using a quantikine mouse immunoassay kit (R & D Systems, Minneapolis, MN) according to the manufacturer’s protocol. EGF concentrations were calculated from a standard curve ranging from 0 to 500 pg/mL. The intra- and inter assay coefficients of variations are <10%, and the sensitivity of the assay is 0.95 pg/mL. Data from ileum homogenates were standardized using total cellular protein levels.
Quantitative real-time PCR
Total RNA was extracted using RNA Pro solution (MP Bio, Solon, OH) and allowed to digest for 5 min at room temperature. The samples were transferred to microtubes containing ceramic beads and placed in a FastPrep-24 instrument (MP Bio) for 40 s. After addition of chloroform, the samples were vortexed for 30 s and centrifuged at 13,000 rpm at 4°C for 20 min. The upper aqueous phase was transferred to a clean Eppendorf tube containing 0.5 mL of icecold 100% ethanol. The samples were placed in a -20°C freezer overnight to precipitate the RNA. After precipitation, the samples were centrifuged at 13,000 rpm at 4°C for 20 min, and the resulting pellet was washed in 75% ethanol/water and re-dissolved in 100 γL nuclease-free water. Cleanup of the RNA was performed using RNEasy mini cleanup kits (Qiagen, Valencia, CA). One microgram of total RNA was reversely transcribed with random primers according to the manufacturer’s protocol. The cDNA was diluted and the real-time PCR was performed using RT2 Profiler Rat NO Signaling PCR Array System (SABiosciences, Frederick, MD). The real-time PCR arrays were done on 96-well plates pre-coated with 84 genes in the cytokine, TLR, and oxidative stress signaling pathways. An aliquot of 25 γL was added to each well. Five different control genes present on the PCR array plates were used to normalize the mRNA expression of the genes studied. Calculations were made by uploading the real-time amplification data into the SABiosciences RT2 Profiler PCR Array Data Analysis web portal. Quantitative PCR was based on the cycle threshold (Ct) value. A gene was considered not detectable if the Ct value was >32.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine differences among the groups for normally-distributed data, and Kruskal-Wallis test was used for non-normally distributed data following Bartlett’s test for equality of variances. Post hoc analysis was performed using the Tukey and Student-Newman-Keuls tests for significance. Significance was set at p<0.05 and data are reported as mean ± SEM. All analyses were two-tailed and performed using SPSS (SPSS, Inc. Chicago IL).
Results
Effects on growth
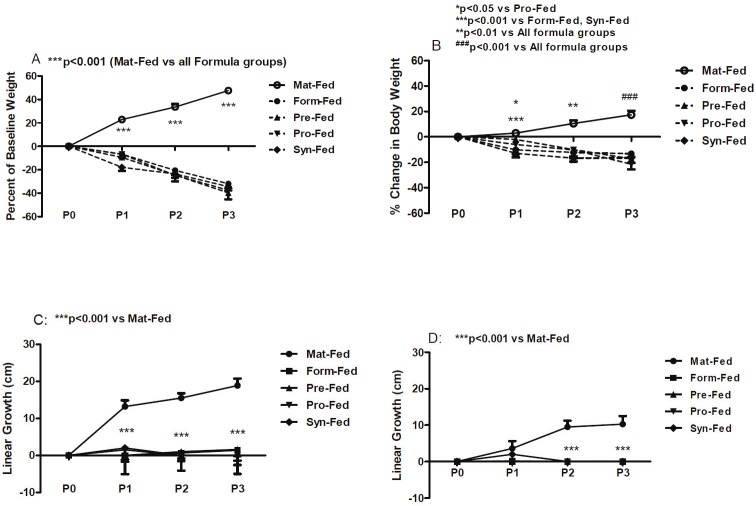
Randomization at birth resulted in comparable mean total body weight (grams) and linear growth (cm) in all groups. Percentage change in body weight is shown in the Figure 1A and 1B. In room air (A), weight accretion in the maternally-fed pups was progressive and significant from P0 to P3. In contrast, the formula-fed groups demonstrated a reversed effect despite supplementation. In hyperoxia-hypoxia (B), the response was similar, but attenuated. Percentage change in linear growth is shown in Figure 1C and 1D. In room air (C), there were marked and significant increases in percentage change in linear growth from P0 to P3 in the maternally-fed group. Linear growth in all formula groups did not appreciable change and was significantly lower than the maternally-fed group. In hyperoxia/hypoxia (D) there were no significant differences between maternally-fed and supplemental groups at P0 or P1. However, at P2 and P3, percentage change in linear growth declined significantly in the formula-fed groups.
Figure 1.
Percentage change in body weight (A and B) and linear growth (C and D) in maternally-fed (Mat-Fed) neonatal rats; and rats gavaged with formula (Form-Fed); formula supplemented with probiotics (Pro-Fed); formula supplemented with prebiotics (Pre-Fed); and formula supplemented with synbiotics (Syn-Fed). n=18 pups/group. A and C: (room air); B and D: (hyperoxia/hypoxia) groups. One-way analysis of variance (ANOVA) was used to determine differences among the groups for normally-distributed data, and Kruskal-Wallis test was used for non-normally distributed data following Bartlett’s test for equality of variances. Post hoc analysis was performed using the Tukey and Student-Newman-Keuls tests for significance. Significance was set at p<0.05 and data are reported as mean ± SEM.
Cytokine levels
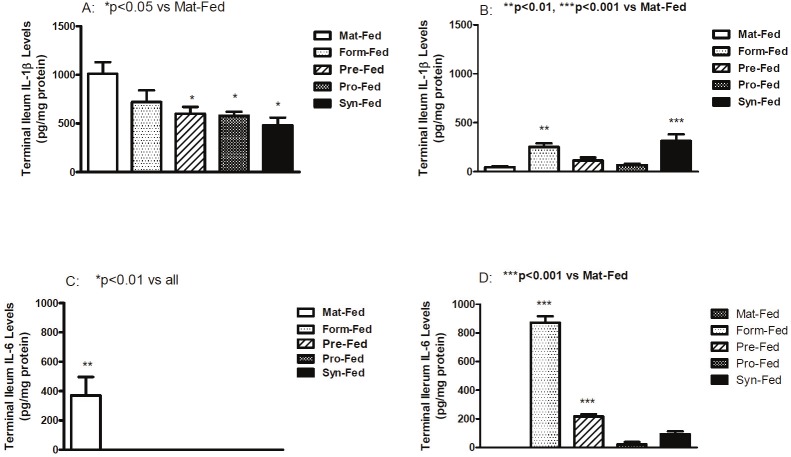
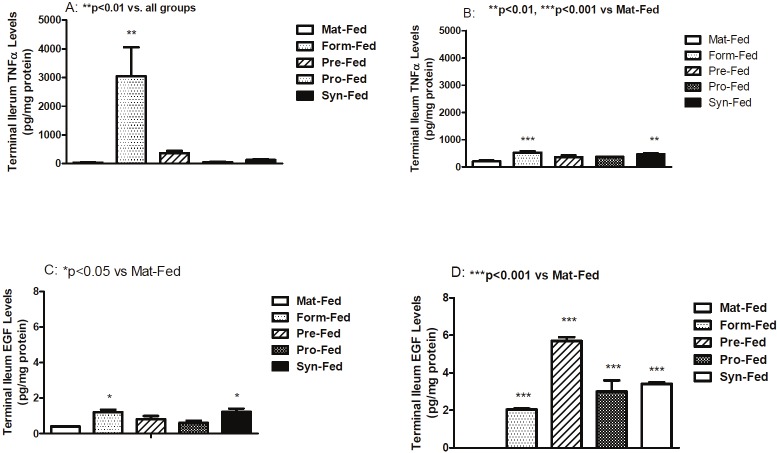
Terminal ileum IL-1β levels are presented in Figure 2A and 2B. Room air levels were generally higher than hyperoxia/hypoxia. In room air (A), terminal ileum IL-1β levels were significantly decreased in the supplemented formula groups compared with the maternally fed group. In hyperoxia-hypoxia (B), IL-1β was significantly elevated in formula fed and synbiotic fed groups. IL-6 levels are presented in Figure 2C and 2D. IL-6 levels were low to undetected in all room air formula-fed groups (C). However, in hyperoxia-hypoxia (D), IL-6 levels were significantly elevated in un-supplemented formula and prebiotics compared to maternally-fed pups. TNFα levels are presented in Figure 3A and 3B. In room air (A), TNFα levels were significantly increased in formula-fed group only whereas in hyperoxia/hypoxia (B), TNFα levels were significantly elevated in un-supplemented formula and synbiotic groups. EGF levels are presented in Figure 3C and 3D. In room air (C) EGF levels were generally lower than hyperoxia/hypoxia (D) and were significantly higher in the un-supplemented and synbiotic groups compared to maternally fed group. In contrast, EGF levels were significantly higher in all formula-fed hyperoxia-hypoxia compared to maternally-fed groups.
Figure 2.
Mean IL-1β and IL-6 levels in terminal ileum homogenates. Groups are as described in Figure 1.
Figure 3.
Mean TNFα and EGF levels in terminal ileum homogenates. Groups are as described in Figure 1.
Effects on pro-inflammatory cytokine genes
Expression of pro-inflammatory cytokine genes in room air and hyperoxia-hypoxia in terminal ileum of rat pups are depicted in Table 1. Data are folded expression change from maternally-fed. In general, there was up-regulation of pro-inflammatory cytokine genes in all room air formula-fed groups. Supplementation downregulated most pro-inflammatory cytokines, but the most robust were IFNα (27-fold) and IL-1β (29-fold) seen with probiotics. In hyperoxia/hypoxia (Table 2) similar down-regulation of pro-inflammatory cytokines were noted with supplementation. Other remarkable findings were marked down regulation of IL-6 (12-fold) with prebiotics, IFNβ (15-fold) with probiotics and IFN-γ (18-fold) with synbiotics in hyperoxia/hypoxia.
Table 1.
Expression of Pro-inflammatory Cytokine Genes in the Terminal Ileum of Neonatal Rats Raised in Room Air (RA) and hyperoxia/hypoxia (H/H). Data are fold expression difference from Maternally-Fed RA or H/H.
| Genes of Interest | Un-supplemented Formula | Prebiotics | Probiotics | Synbiotics | ||||
|---|---|---|---|---|---|---|---|---|
| RA | H/H | RA | H/H | RA | H/H | RA | H/H | |
| COX-2 | 45.0 | -1.9 | -4.4 | -2.1 | -2.5 | 2.4 | -1.4 | -2.7 |
| IFNα | 8.2 | 24.1 | -1.8 | -1.9 | -27.1 | -1.3 | -8.1 | -3.0 |
| IFNβ | 104.3 | 6.9 | 5.8 | -3.4 | -2.6 | -14.5 | 1.3 | -3.0 |
| IFNγ | 8.8 | 1.4 | 1.8 | -2.5 | -1.0 | -5.0 | -3.3 | -18.4 |
| IL12α | -3.0 | -3.2 | 1.0 | -9.8 | -6.7 | -2.6 | -3.6 | -4.4 |
| IL-1r1 | 22.3 | -3.3 | -1.4 | -1.8 | -3.8 | -3.2 | 1.2 | -2.4 |
| IL-1α | 2.9 | 1.3 | -4.9 | -4.5 | -28.6 | 3.7 | -2.6 | 2.7 |
| IL-1β | 6.3 | 11.7 | -7.7 | -5.8 | -2.5 | 1.2 | -4.4 | -2.4 |
| IL-6 | 26.4 | 6.2 | 17.3 | -11.6 | -3.3 | 1.9 | -3.3 | -2.9 |
| NFkB | 11.7 | 6.1 | 2.6 | -1.8 | -1.7 | -2.0 | -1.7 | -2.7 |
| TNFα | 45.6 | 1.5 | 3.4 | -3.4 | -2.4 | -6.7 | -3.2 | -6.3 |
Genes of interest are: COX, cyclooxygenase; IFN, interferon; IL, interleukin; NFkB, nuclear factor kappa B; TNF, tumor necrosis factor
Table 2.
Expression of Toll-like Receptor (TLR) Genes in the Terminal Ileum of Neonatal Rats Raised in Room Air (RA) and hyperoxia/hypoxia (H/H). Data are folded expression difference from Maternally-Fed RA or H/H.
| Genes of Interest | Un-supplemented Formula | Prebiotics | Probiotics | Synbiotics | ||||
|---|---|---|---|---|---|---|---|---|
| RA | H/H | RA | H/H | RA | H/H | RA | H/H | |
| TLR-1 | 22.7 | -7.3 | -7.9 | -3.7 | 1.03 | 1.0 | -71.2 | -1.8 |
| TLR-2 | 14.1 | -2.7 | -4.6 | -2.3 | -1.5 | 1.9 | -14.6 | -1.2 |
| TLR-3 | 1.1 | -19.1 | -6.9 | -17.4 | -10.7 | -478.4 | -1.8 | -1.4 |
| TLR-4 | -2.1 | -5.8 | -1.3 | -2.3 | -1.0 | -1.3 | -1.2 | -3.3 |
| TLR-5 | 9.8 | -4.0 | -1.8 | 1.3 | 1.4 | 1.2 | -3.1 | -2.4 |
| TLR-6 | 16.1 | 2.9 | -3.4 | -1.1 | 2.3 | 2.0 | -4.9 | -1.2 |
| TLR-7 | 15.1 | 2.2 | -1.3 | 1.4 | -7.5 | 1.7 | -6.8 | -1.4 |
| TLR-9 | 6.2 | 1.5 | -3.4 | -4.4 | 1.2 | -2.6 | -3.1 | -5.7 |
Effects on Toll-like receptor genes
Expression of Toll-like receptor genes in room air and hyperoxia-hypoxia in terminal ileum of rat pups are depicted in Table 2. In room air, TLR genes showed almost uniform up-regulation with unsupplemented formula, except for TLR-4. Supplementation generally led to down-regulation of TLR genes. Most prominent changes were down-regulation of TLR-3 (11-fold) and -7 (8-fold) with probiotics, TLR-1 (8-fold) and TLR-3 (7-fold) with prebiotics, and TLR-1 (71-fold), TLR-2 (15-fold) and TLR-7 (7-fold) with synbiotics. In hyperoxia/hypoxia, all the groups showed a general tendency for down-regulation of TLR genes irrespective of whether the formula is supplemented or not. The most robust effects were noted with prebiotics and probiotics which resulted down-regulated of TLR-3 (17-fold and 478-fold, respectively).
Regulation of oxidative stress
Genes involved in regulation of oxidative stress are depicted in Table 3. We examined catalase, glutathione peroxidase (GPX) 1-7 and superoxide dismutase (SOD) 1-3 genes in terminal ileum. In room air, all genes were down-regulated in un-supplemented formula groups with the most robust effects seen with catalase (2096-fold), GPX-5 (1546-fold), GPX-6 (645-fold), GPX-7 (2288-fold), and SOD-3 (101-fold). Similar responses were noted in the supplemented groups, but the effects were substantially attenuated. In hyperoxia/hypoxia, only SOD-3 appeared to respond positively with supplementation.
Table 3.
Expression of Oxidative Stress Genes in the Terminal Ileum of Neonatal Rats Raised in Room Air (RA) and hyperoxia/hypoxia (H/H). Data are folded expression difference from Maternally-Fed RA or H/H.
| Genes of Interest | Un-supplemented Formula | Prebiotics | Probiotics | Synbiotics | ||||
|---|---|---|---|---|---|---|---|---|
| RA | H/H | RA | H/H | RA | H/H | RA | H/H | |
| CAT | -2096 | -1.5 | -634 | -1.2 | -634 | -3.9 | -2051 | -2.0 |
| GPX1 | -3 | -1.4 | -1 | 1.2 | 1 | 2.0 | -2 | -1.8 |
| GPX2 | -22 | 11.5 | -2 | 8.4 | -6 | 24.5 | -8 | 10.5 |
| GPX3 | -28 | -1.5 | 1 | -2.6 | 9 | 1.4 | 12 | -9.0 |
| GPX4 | -29 | 2.3 | -4 | 1.6 | 3 | -1.5 | -7 | 1.2 |
| GPX5 | -1546 | -2.4 | -355 | -2.4 | -455 | -26.0 | -542 | -10.7 |
| GPX6 | -645 | -1.2 | -140 | -1.4 | -191 | -14.6 | -162 | 3.7 |
| GPX7 | -2288 | -2.1 | -80 | -1.2 | -120 | -1.6 | -174 | -2.8 |
| SOD-1 | -15 | 1.2 | 12 | 1.1 | 11 | 1.1 | 15 | 1.4 |
| SOD-2 | -28 | 1.5 | -13 | 1.9 | -18 | 2.5 | -17 | 1.8 |
| SOD-3 | -101 | -3.0 | -7 | -1.3 | -29 | -1.4 | -19 | -4.7 |
Genes of interest are: Cat, catalase; GPX, glutathione peroxidase; SOD, superoxide dismutase
Discussion
In neonatal intensive care, extremely premature infants who are prone to develop NEC often experience extremes of oxygen saturations leading to oxidative stress, a well known risk factor for NEC [15-17]. The present study tested the hypothesis that probiotics, prebiotics and/or synbiotics supplementation are effective for reducing formula-induced inflammation and oxidative stress in the terminal ileum. The rat model has advanced our understanding of NEC pathogenesis [18]. Gross and microscopic changes similar to human necrotizing enterocolitis can be easily elicited in neonatal rat pups with various insults as precipitating factors. Combined treatment of formula gavage with intermittent episodes of either cold or hypoxic stress [19], stress induced by formula feeding associated with asphyxia and/or exogenous bacterial colonization [20], and combination of asphyxia and cold stress over 4 days [21] are some examples of stress used to precipitate NEC in rat models. These insults are not clinically relevant. We, as well as others showed in our previous study that human formula feeding alone in rat pups produces various degrees of inflammatory changes and shares many histopathological and clinical features of NEC seen in human preterm newborns [9,22].
We used live, freeze-dried yeast cells of the species Saccharomyces Boulardii lyo as probiotic because it is well known and well accepted probiotic used world wide for more than 50 years. The type of probiotics is not uniform. Studies used Saccharomyces [23], bifidobacteria [24-26] or probitic mixture-Bifidobacteria infantis, Streptococcus thermophilus, and Bifidobacteria bifidus or probiotic mixture Infloran -which is a mixture of Lactobacillus Acidophilus and Bifidobacterium Infantis [27] in animal or human studies. Both GOS and FOS are found in breast milk. A mixture of 90% galacto-oligosaccharides and 10% fructo-oligosaccharides was composed to mimic the molecule size distribution of human milk oligosaccharides [28,29].
We found major differences in the growth accretion rate between room air and hyperoxia/hypoxia exposure even in maternally-fed pups. These findings indicate that exposure to supraphysiologic levels of oxygen with hypoxic insults, although brief, may reduce the feeding capacity of pups, maternal milk production, and/or growth-promoting effects of maternal milk. Furthermore, percentage increase in body length was delayed at least one day in hyperoxia/hypoxia, further demonstrating the growth retarding effect of oxidative stress. Poor weight gain in the formula group was due to less than adequate nutrition, but, exposure to hyperoxa/hypoxia ameliorated the dramatic weight loss seen in room air. The patho-physiology of NEC involves a complex interaction of pro- (IL-6, IL-8, IL-12, IL-18, interferon gamma, TNF-α, and PAF) and anti-inflammatory (IL-1ra, IL-6, IL-10, IL-11) mediators [30-33]. A large number of different cytokines have been found to be involved in bowel inflammation and these are presumed to be the source for any elevations in circulating levels. IL-1 is a cytokine released from macrophages and other antigen-presenting cells. IL-1 promotes the inflammatory response, is stimulated by TNFα [34], and is often attributed to the onset of fever, increased endothelial leukocyte adhesion, phagocyte activation, and lymphocyte co-stimulation [35]. IL-1 is significantly increased during reperfusion [36]. Due to these properties, IL-1 has been noted to play a pivotal role in the systemic inflammatory response syndrome [35]. In room air, the levels of IL-1β were noticeably higher than that in hyperoxia/hypoxia and supplementation resulted in decreased IL-1β levels. This is most likely due to organ failure and intestinal ischemia. The same may be true for lower levels found in the hyperoxia/hypoxia groups. However, lower IL-1β in the prebiotics and probiotics supplemented hyperoxia/hypoxia groups may suggest possible beneficial effects. IL-6 and its associated receptors are expressed by intestinal endothelium, macrophages, and helper T cells. IL-6 is stimulated by a variety of other pro-inflammatory cytokines, including TNF-α and IL-1β [37]. During sepsis or a systemic inflammatory response, there is an increase in enterocyte-secreted IL-6, which is augmented by bacteria, endotoxins, and other cytokines [38-40]. IL-6 has been shown to be protective for recovery from intestinal ischemia/reperfusion injury [41,42], and prevent intestinal cell death [43]. In our study, IL-6 was not detected in the room air formula groups suggesting intestinal damage and possibly ischemia. Prolonged exposure to hyperoxia/hypoxia may have opposite effects that are attenuated with probiotics, prebiotics or synbiotics. In room air, supplementation reduced the inflammatory response of increased TNFα seen in the un-supplementated group. This effect was abolished in the symbiotic group exposed to hyperoxia/hypoxia. EGF is known to play significant role in intestinal mucosal repair, intestinal epithelial healing, regeneration, and permeability and suggested to be beneficial in treatment of NEC [44]. EGF was lower in all room air than hyperoxia/hypoxia groups suggesting an association with oxidative stress. Higher levels in the formula groups suggest an attempt against injury.
Both pro- and anti-inflammatory cytokines genes were up-regulated in our model of intestinal inflammation. How nonpathogenic bacteria activate epithelial signaling pathways is a topic of intense investigation. Classically, pathogens are monitored by cellular pattern recognition receptors such as TLRs. Probiotics also express TLR ligands, but in the context of intact barrier function, do not cause overt cellular inflammation. TLR ligands and other bacterial products clearly affect epithelial signaling, gene transcription and mediate cytoprotective effects, and TLR signaling is clearly necessary for the overall integrity of the mucosa [45-48]. In our study, TLR receptor genes in terminal ileum were down-regulated in both probiotics and synbiotics. Down-regulation of TLRs indicates protection of intestinal mucosa. The protective effect of synbiotics was most robust. SOD-1 and -2 may be the main enzymes that regulate oxidative stress in our model of NEC. Several limitations of our study must be acknowledged including small sample size, choice of human formula to achieve bowel inflammation, and the microbial content in the small bowel was not determined. Various studies have used different probiotics which adds to the confusion. However we used S. Boulardii which is the most widely used probiotic in humans over half a century. We did not study the correlation between the levels of cytokines to the severity of inflammation in the terminal ileum and we did not examine blood cultures to correlate with elevated cytokines. Notwithstanding, our study demonstrates that supplementation with probiotics, prebiotics, or synbiotics may provide protection against the biochemical and molecular inflammatory responses to formula-induced bowel inflammation.
In summary, our study is the first to examine comparative effects of probiotics, prebiotics and synbiotics on inflammatory markers and genes that regulate inflammation and oxidative stress in the terminal ileum of formula-induced bowel inflammation. The combination of suboptimal nutrition due to removal of breast milk and formula feeding with hyperoxia/hypoxia may increase susceptibility to oxidative stress, inflammation, and NEC. Probiotics, prebiotics and synbiotics are protective against oxidative stress and inflammation, but their efficacy may be reduced when administered during hyperoxia/hypoxia insults.
Acknowledgement
The authors acknowledge and thank the staff of the Department of Laboratory Animal Research, State University of New York, and Brooklyn, NY for their assistance and technical support.
This work was made possible through a grant from the Department of Pediatrics, SUNY Downstate Medical Center, Brooklyn, NY. Grant # 09- 397-08.
References
- 1.Srinivasan PS, Brandler MD, D’Souza A. Necrotizing enterocolitis. Clin Perinatol. 2008;35:251–272. doi: 10.1016/j.clp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32:100–106. doi: 10.1053/j.semperi.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee SH. Basic and translational understanding of microbial recognition by toll-like receptors in the intestine. J Neurogastroenterol Motil. 2011;17:28–34. doi: 10.5056/jnm.2011.17.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R Jr. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 7.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 8.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza A, Fordjour L, Ahmad A, Cai C, Kumar D, Valencia G, Aranda JV, Beharry KD. Effects of probiotics, prebiotics, and synbiotics on messenger RNA expression of caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Pediatr Res. 2010;67:526–531. doi: 10.1203/PDR.0b013e3181d4ff2b. [DOI] [PubMed] [Google Scholar]
- 10.Vandenplas Y, Veereman-Wauters G, De Greef E, Peeters S, Casteels A, Mahler T, Devreker T, Hauser B. Probiotics and prebiotics in prevention and treatment of diseases in infants and children. J Pediatr. 2011;87:292–300. doi: 10.2223/JPED.2103. [DOI] [PubMed] [Google Scholar]
- 11.Stenger MR, Beber KM, Giannone PJ, Nankervis CA. Probiotics and prebiotics for the prevention of necrotizing enterocolitis. Curr Infect Dis Rep. 2011;13:13–20. doi: 10.1007/s11908-010-0156-6. [DOI] [PubMed] [Google Scholar]
- 12.Joint FAO/WHO Working group. Guidelines for evaluation of probiotics in food: report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food, London, Ontario, Canada 2002. Available at http://www.fermented-foods.net/wgreprort2.pdf. accessed 19. [Google Scholar]
- 13.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 14.Isolauri E, Kirjavainen PV, Saminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50:54–59. doi: 10.1136/gut.50.suppl_3.iii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F, Iacoponi F, Capitani S, Buonocore G. Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum Dev. 2010;86:241–244. doi: 10.1016/j.earlhumdev.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Guven A, Gundogdu G, Vurucu S, Uysal B, Oztas E, Ozturk H, Korkmaz A. Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2009;44:1730–1735. doi: 10.1016/j.jpedsurg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Miller MJ, McNeill H, Mullane KM, Caravella SJ, Clark DA. SOD prevents damage and attenuates eicosanoid release in a rabbit model of necrotizing enterocolitis. Am J Physiol. 1988;255:G556–565. doi: 10.1152/ajpgi.1988.255.5.G556. [DOI] [PubMed] [Google Scholar]
- 18.Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. 2008;1:94–98. doi: 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77:687–690. [PubMed] [Google Scholar]
- 20.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 21.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res. 2003;53:426–433. doi: 10.1203/01.PDR.0000050657.56817.E0. [DOI] [PubMed] [Google Scholar]
- 22.Fordjour L, D'Souza A, Cai C, Ahmad A, Valencia G, Kumar D, Aranda JV, Beharry KD. Comparative effects of probiotics, prebiotics, and synbiotics on growth factors in the large bowel in a rat model of formula-induced bowel inflammation. J Pediatr Gastroenterol Nutr. 2010;51:507–513. doi: 10.1097/MPG.0b013e3181df5ff2. [DOI] [PubMed] [Google Scholar]
- 23.Akisu M, Baka M, Yalaz M, Huseyinov A, Kultursay N. Supplementation with Saccharomyces boulardii ameliorates hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. Eur J Pediatr Surg. 2003;13:319–323. doi: 10.1055/s-2003-43580. [DOI] [PubMed] [Google Scholar]
- 24.Butel MJ, Roland N, Hibert A, Popot F, Favre A, Tessedre AC, Bensaada M, Rimbault A, Szylit O. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. 1998;47:391–399. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 25.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R Jr. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 26.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 28.Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38:S291–294. doi: 10.1016/S1590-8658(07)60013-9. [DOI] [PubMed] [Google Scholar]
- 29.Boehm G, Fanaro S, Jelinek J, Stahl B, Marini A. Prebiotic concept for infant nutrition. Acta Paediatr Suppl. 2003;91:64–67. doi: 10.1111/j.1651-2227.2003.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 30.Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J Pediatr. 1990;116:960–964. doi: 10.1016/s0022-3476(05)80661-4. [DOI] [PubMed] [Google Scholar]
- 31.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counter-inflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–771. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 32.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Upregulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res. 2002;51:733–739. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 34.Minekawa R, Takeda T, Sakata M, Yamamoto T, Hayashi M, Tasaka K, Murata Y. Human breast milk suppresses the transcriptional regulation of IL-1[beta] induced NF-[kappa] B signaling in human intestinal cells. Am J Physiol Cell Physiol. 2004;287:C1404–1411. doi: 10.1152/ajpcell.00471.2003. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA, Gelfand JA, Wolff SM. Anticytokine strategies in the treatment of the systemic inflammatory response syndrome. JAMA. 1993;269:1829–1835. [PubMed] [Google Scholar]
- 36.Spanos CP, Papaconstantinou P, Spanos P, Karamouzis M, Lekkas G, Papaconstantinou C. The effect of L-arginine and aprotinin on intestinal ischemia-reperfusion injury. J Gastrointes Surg. 2007;11:247–255. doi: 10.1007/s11605-007-0102-6. [DOI] [PubMed] [Google Scholar]
- 37.Romagnoli C, Frezza S, Cingolani A, Puopolo M, De Carolis MP, Vento G, Antinori A, Tortorolo G. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160:345–350. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 39.Goepfert AR, Andrews WW, Carlo W, Ramsey PS, Cliver SP, Goldenberg RL, Hauth JC. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol. 2004;191:1375–1381. doi: 10.1016/j.ajog.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 40.Harris MC, Costarino AT Jr, Sullivan JS, Dulkerian S, McCawley L, Corcoran L, Butler S, Kilpatrick L. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr. 1994;124:105–111. doi: 10.1016/s0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res. 1998;79:39–46. doi: 10.1006/jsre.1998.5385. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Sun X, Pritts TA, Wong HR, Hasselgren PO. Induction of the stress response increases interleukin-6 production in the intestinal mucosa of endotoxaemic mice. Clin Sci (Lond) 2000;99:489–496. [PubMed] [Google Scholar]
- 43.Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–196. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- 44.Maynard MA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A, Dvorak B. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G614–622. doi: 10.1152/ajpgi.00076.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, Gewirtz AT, Neish AS. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G96–108. doi: 10.1152/ajpgi.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 48.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]