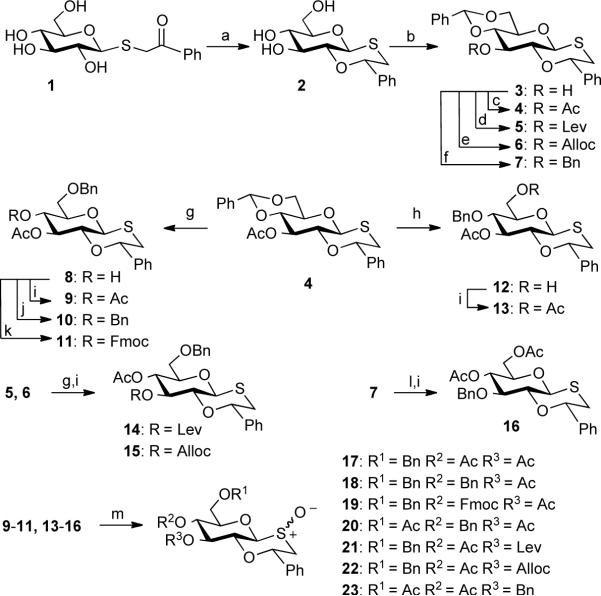
Scheme 2.
Synthesis of 1,4-oxathiane protected donors
a) TMS2O, TMSOTf, 0 °C, 30 min, then Et3SiH, 3 h (74%); b) PhCH(OMe)2, CSA, reduced pressure, DMF, 50 °C,18 h (87%); c) Ac2O, Py; d) Levulinic acid, DCC, DMAP, DCM (79%); e) AllocCl, TMEDA, DMAP, DCM (87%); f) BnBr, NaH, DMF (79%); g) Et3SiH, TfOH, DCM, −78 °C; h) Et3SiH, PhBCl2, −78 °C; i) Ac2O, Py(9: 85%, g, i 2 steps; 13: 78%, h, i 2 steps; 14: 79%, g, i 2 steps; 15: 92%, g, i 2 steps; 16: 84%, l, i 2 steps); j) Ag2O, BnBr (10: 55%, g, j 2 steps); k) FmocCl, Py/DCM = 1/1 (v/v) (11: 66%, g, k 2 steps); l) EtSH, TsOH, DCM; m) mCPBA, DCM, −78 °C (17: 92%; 18: 96%; 19: 98%; 20: 93%; 21: 98%; 22: 82%; 23: 96%).