Table 1.
Stereoselective glycosylations between donor 17 and various acceptors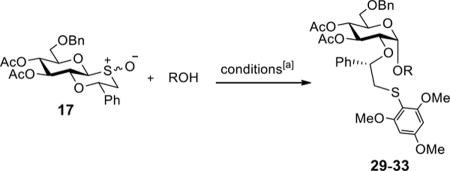
| Entry | ROH | Product | Yield[b] | α:p[c] |
|---|---|---|---|---|
| 1 | 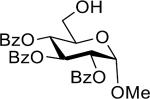 |
29 | 91% | >15:1 |
| 24 | ||||
| 2 | 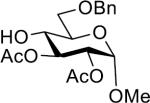 |
30 | 62% | >25:1 |
| 25 | ||||
| 3 |  |
31 | 89% | α only |
| 26 | ||||
| 4 | 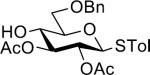 |
32 | 67% | >15:1 |
| 27 | ||||
| 5 | 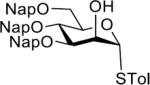 |
33 | 72% | α only |
| 28 |
1,3,5-Trimethoxybenzene, Tf2O, DTBMP, molecular sieves 4 Å, −10 °C, 30 min, then add acceptor, −40 °C to room temperature, 16 h.
Isolated yields of the α/β mixture of disaccharide products.
The α/β ratios were determined by the integration of key signals in the 1H NMR spectra of the disaccharide products after purification by LH-20 size exclusion chromatography.
