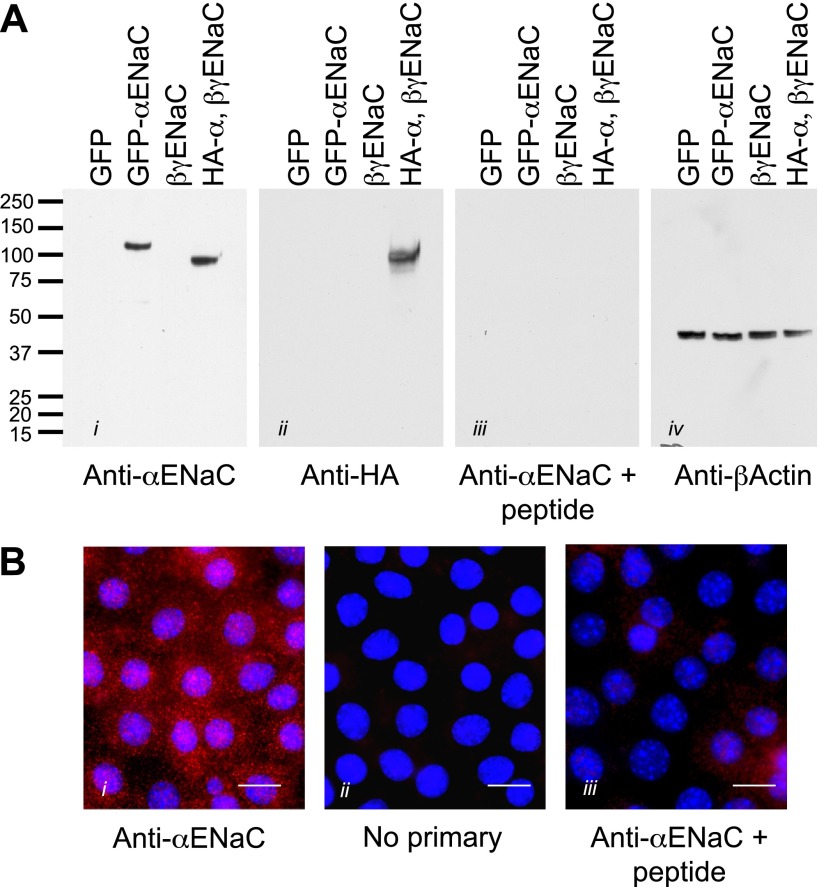
Fig. 1.
Characterization of a novel anti-α-epithelial sodium channel (ENaC) antibody. A: to test the specificity of the affinity-purified antibody, Fisher rat thyroid (FRT) cells were transiently transfected with the following constructs: green fluorescent protein (GFP) alone, GFP-tagged mouse α-ENaC, untagged mouse β,γ-ENaC only, and hemagglutinin (HA)-tagged mouse α-ENaC with untagged β,γ-ENaC. Whole cell lysates from these samples were loaded onto gels, and proteins were resolved by SDS-PAGE. Blots were probed with i) the α-ENaC antibody (1:1,000), ii) anti-HA antibody (1:1,000), iii) the α-ENaC antibody preincubated with the immunizing peptide (1 μg/μl), and iv) anti-β actin (1:1,000) as a loading control. From the detected bands, it is clear that the antibody recognizes the full-length α-ENaC and not β- or γ-ENaC. The addition of a GFP tag shifted the apparent weight up by ∼20 kDa as expected, and use of the anti-HA antibody produced a band at the same weight, indicating that the antibody is recognizing α-ENaC specifically. In addition, preincubation with the immunizing peptide eliminated the specific signal. It is therefore likely that this antibody is specific for α-ENaC in the mpkCCD cells. B: to demonstrate the specificity of fluorescent immunolabeling, mpkCCD cells cultured on filters were fixed (as described in materials and methods) and permeabilized, and indirect immunofluorescent labeling was performed using an Alex 568 anti-rabbit secondary antibody (Invitrogen) at 1:2,000 dilution with the following conditions: i) α-ENaC as the primary antibody, ii) no primary antibody (to test for nonspecific secondary labeling), and iii) α-ENaC antibody preincubated with immunizing peptide (as for the Western blots in A). Bars = 10 μm.