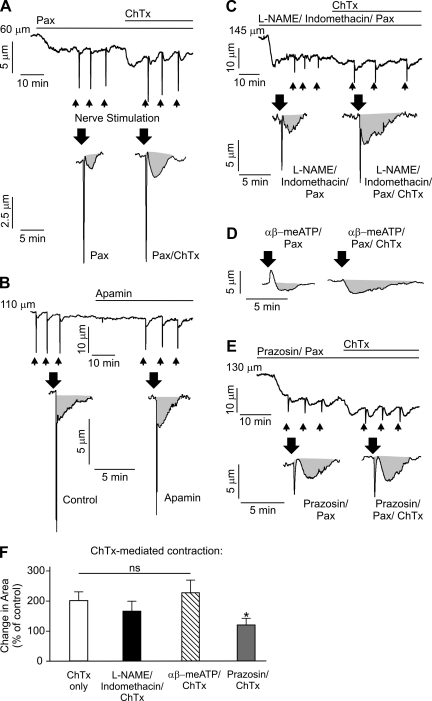
Fig. 4.
Intermediate-conductance (IK) channels oppose nerve-induced constriction via a mechanism that is dependent on vascular smooth muscle cell α-adrenergic receptor activation and independent of nitric oxide synthase/cyclooxygenase. A–E: representative diameter changes in pressurized (80 mmHg, 166 ± 25-μm maximal diameter and 129 ± 23-μm resting diameter, n = 34 arteries) third-order mesenteric arteries in response to EFS (indicated by arrows). Gray areas indicate area under the curve, which was calculated as an integral of the constriction. A: application of 100 nM charybdotoxin (ChTx) in the presence of 1 μM paxilline (Pax) increased constriction to identical nerve stimulation by twofold (n = 6 arteries). B: application of 300 nM apamin did not cause vasoconstriction (1.4 ± 1.0%, n = 5 arteries) and did not significantly alter nerve-induced vasoconstriction (P > 0.05, n = 5 arteries). This increase in EFS-induced constriction produced by inhibition of IK channels with ChTx was not significantly effected by pretreatment with 100 μM N-nitro-l-arginine methyl ester (l-NAME)-10 μM indomethacin-1 μM Pax (n = 5 arteries; P > 0.05; C) or 10 μM αβ-meATP-1 μM Pax (n = 5 arteries, P > 0.05; D) but was abolished by pretreatment with 500 nM prazosin-1 μM Pax (n = 5 arteries, *P < 0.05; E). Although 10 μM αβ-meATP completely abolished peak constriction in response to EFS, its effects on the area under the curve were minimal. F: summary graph illustrating the changes in luminal diameter upon EFS. For all experiments with ChTx, arteries were first incubated with 1 μM Pax to block smooth muscle large-conductance channels. Control corresponds to pretreatment of vessels with the indicated pathway inhibitors (in the presence of Pax).