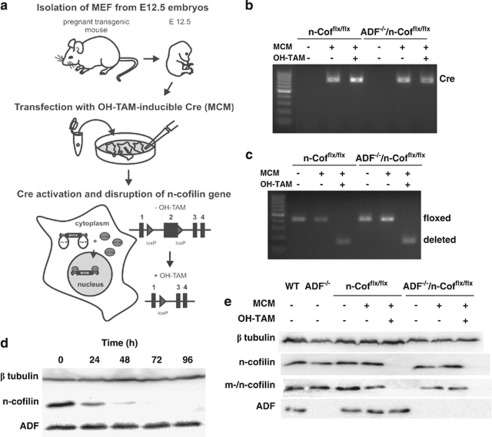
Figure 2.
Preparation of MEFs lacking ADF, n-cofilin, or both proteins. (a) Experimental outline for the generation of ADF- and/or n-cofilin-deficient MEFs. MEFs were isolated from E12.5 embryos and passaged until spontaneous immortalization. Disruption of the floxed n-cofilin gene was obtained by stable transfection of MEFs with MCM and subsequent OH-TAM treatment. (b) PCR results confirming the expression of MCM in transfected n-Cofflx/flx and ADF−/−/n-Cofflx/flx MEFs. (c) PCR results confirming efficient disruption of the n-cofilin gene (deleted) in MCM-transfected and OH-TAM-treated MEFs. In contrast, the n-cofilin gene is undisrupted (floxed) in non-transfected MEFs, as well as in MCM-transfected MEFs not treated with OH-TAM. (d) Decline of n-cofilin protein levels in MCM-transfected n-Cofflx/flx MEFs upon OH-TAM treatment. At 72 h after the onset of OH-TAM treatment, n-cofilin protein was undetectable. ADF expression was unaltered in n-cofilin-deficient MEFs; β-tubulin was used to control protein loading. (e) Characterization of the four different MEF lines. No change in n-cofilin protein level was detected upon transfection with MCM. An antibody recognizing n-cofilin and m-cofilin was used to demonstrate that MCM-transfected and OH-TAM-treated n-Cofflx/flx and ADF−/−/n-Cofflx/flx MEFs do not express m-cofilin. Protein load was controlled using a β-tubulin antibody