Summary
This article focuses on the treatment of neurovascular diseases, in particular brain arteriovenous malformations (BAVMs), with radiosurgery. The target group for this review is physicians who manage patients with neurovascular diseases, but are not actively engaged in radiosurgery.
Radiosurgery for BAVMs is an established treatment with clearly defined risks and benefits. The efficacy of radiosurgery for dural arteriovenous shunts (DAVSs) is probably similar but the treatment has not yet gained the same acceptance. Radiosurgical treatment of cavernomas (cavernous hemangiomas) remains controversial.
Well founded predictive models for BAVM radiosurgery show:
• The probability of obliteration depends on the dose of radiation given to the periphery of the BAVM.
• The risk of adverse radiation effects depends on the total dose of radiation, i.e. the amount of energy imparted into the tissue. The risk is greater in centrally located lesions. The risk of damage to brainstem nucleii and cranial nerves must be added to the risk predicted from current outcome models.
• The risk of hemorrhage during the time span before obliteration depends on the BAVM volume, the dose of radiation to the periphery of the lesion and the age of the patient. Central location is a probably also a risk factor.
Keywords: radiosurgery, gamma knife radiosurgery, dural arteriovenous shunts, intracranial arteriovenous malformations, review
Introduction
Brain arteriovenous malformations (BAVMs) are relatively uncommon. In the western population the prevalence has been estimated to be between 0.06% and 0.11% and the incidence to be between 0.01% and 0.001% 1-9.They are a major cause of hemorrhagic stroke in the young and middle aged population, second only to rupture of an arterial aneurysm. Other neurovascular disease entities that may expose a patient to a risk of intracranial hemorrhage are dural arteriovenous shunts (DAVSs), also called fistulae, and cavernomas.
In adults, DAVSs are about ten times less common than BAVM 10. The incidence of cavernoma in the western population is not very well known, principally due to the low risk of developing neurological symptoms as a result of the disease.
To prevent the neurological sequelae that would most likely result from a future hemorrhagic event, patients with BAVMs or DAVSs (i.e. DAVS with cortical venous drainage) are most often subjected to treatment unless it is anticipated that the risk posed by the preventive action itself cannot be justified. For patients with cavernomas, opinions differ about what is the proper management strategy.
This article focuses on the treatment of neurovascular diseases, in particular BAVMs, with radiosurgery (RS). The author's experience is mostly in Gamma knife radiosurgery (GKRS). The examples and the majority of references come from this irradiation technique.
The target group for this review is physicians who manage patients with neurovascular diseases, but are not actively engaged in RS.
Definitions with comments
Radiation units
Radiosurgery is performed using heavy charged particles (proton beam) or photons with different levels of energy, i.e. X-rays (linear accelerator, LINAC) or gamma-rays (Gammaknife, GK).
Proton beam RS relies on the Bragg-peak effect to minimize the irradiation to the surrounding tissue 11,12. It is performed only at a very limited number of dedicated centers and will not be discussed in detail.
Many LINAC:s have been adapted for stereotaxy, with the radiation source moving around the patients head in arcs or more complicated patterns. High precision can be attained with new equipment utilizing multi-leaf collimators 13. However, no studies on the effect of mechanical wear on their precision has been published to our knowledge.
The Gamma Knife (Elekta Instruments AB, Stockholm, Sweden) is a half sphere of pure iron with a hollow centre. Inside the sphere there are 201 Cobalt-61 sources, focused at an isocenter where the target volume is positioned. Very high precision is possible because of the mechanical stability of the system 14.
The GK is superior to stereotactically adapted LINACs in target conformity, dose gradient and precision but not in dose homogeneity. It is unclear how this affects the outcome. Conformity and dose homogeneity are inversely related to each other, i.e. homogeneous dose distribution implies low conformity. For the same reasons irradiation to the surrounding tissue is higher with LINAC than with the GK.
Radiosurgery or radiotherapy
Radiosurgery refers to external high precision single dose irradiation of a small target volume, typically intracranial 15,16. Rapid dose fall off outside the target volume is inherent in the technique. With the GK in a clinical setting 50% dose fall off over a distance of 5-7 mm is common, and up to 40 % per mm can be achieved. Due to the more homogeneous dose distribution, the dose fall off is less sharp for LINAC and proton beam RS. The dose fall off in combination with high precision (which can, under optimal conditions, be in the sub-mm range throughout the imaging and treatment chain) make it possible to deliver a high dose of radiation to the target volume with an acceptable dose to the surrounding tissues 14. Good conformity between the target volume and the prescription isodose volume is another fundamental characteristic of RS 17.
Spatially fractionated or staged volume RS refers to irradiation of different parts of the target volume during different treatment sessions 18. Typically one part of a complex BAVM is treated first, with a full dose of radiation. The obliteration of the first part facilitates delineation and treatment of the remaining nidus 19. Each session is a separate treatment with a full dose of radiation. In the teratmetn of BAVM the prescription dose (dose to the target periphery) will commonly be in the interval 18-25Gy.
Temporally fractionated RS or hypofractionation is a misnomer for precision radiotherapy, i.e. treatment of the same volume in several sessions with low doses of radiation 20. This technique does not seem to be beneficial in the treatment of BAVMs and can increase the risk for complications without a concomitant increase in the likelihood of positive therapeutic effects 21,22. In a previous report, the long term results of fractionated LINAC irradiation of large BAVMs were dismal 23.
Another scheme for hypofractionation in treatment of large BAVMs is whole target irradiation with reduced dose, with the aim being to treat a second time months or years later, when the lesion may have diminished in flow and volume. Data on the outcome of this strategy are very sparse.
It is clear that multiple treatments with irradiation of a BAVM carry a relatively higher risk of adverse radiation effects as compared to a single treatment, if all other factors are unchanged 24.
Imaging
Accurate imaging is absolutely essential in RS. The geometric distortion in magnetic resonance imaging (MRI), particularly in 3T scanners, can be considerable and not even computed tomography (CT) is completely free of distortion 25-28. Rigorous monitoring of the equipment is crucial 14.
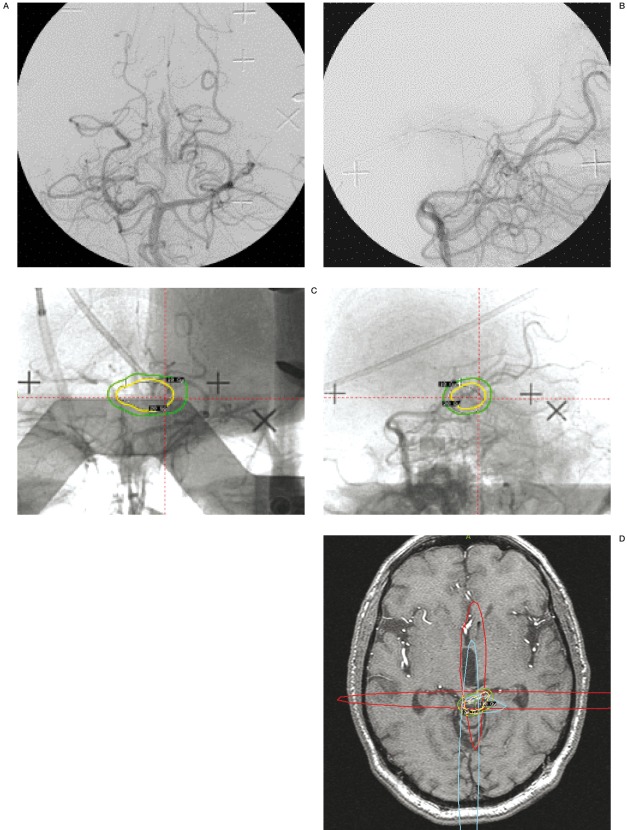
Biplane stereotactic conventional angiography, if necessary with correction of the geometric image distortion originating from the image intensifiers, is the "gold standard" in delineation of the niduses of BAVMs and DAVS. Angiographic data should be merged with MRI or CT to visualize adjacent radiation-sensitive structures and normal brain tissue 28-32. Conventional angiography has the advantages of very high resolution and abundant information on hemodynamics, but is limited by being two dimensional projections of three dimensional structures. MRI and CT provide better volume data but lower spatial resolution and less information about hemodynamics. The initial delineation from conventional angiography is thus adjusted according to the information provided by the MRI or CT (figure 1A-D).
Figure 1.
A) Vertebral angiography, posteroanterior view. A poorly defined BAVM is present in the quadrigeminal cistern, draining into the precentral vein. B) Vertebral angiography, lateral view. The same BAVM is visible. C) Biplane angiography in stereotactic frame. The final dose plan is projected onto the angiographic images. The 20 Gy isodose is depicted in yellow and and the 10 Gy isodose in green. D) The same dose plan has been projected onto the stereotactic MRI. The red and blue lines represent the projections from the stereotactic angiography. Note how the 20 Gy isodose line (yellow) has been shaped to avoid excessive irradiation to the quadrigeminal plate. The dose fall off is 10 Gy within 5 mm (green line). The left superior quadrigeminal body was damaged by the initial hemorrhage and the patient had permanent diplopia. Therefore was accepted a slightly higher than usual radiation dose to the left side of the quadrigeminal plate. The BAVM nidus was not discernible on the MRI, performed without and with Gadolinium and including MR angiography. At angiography two years after the treatment the BAVM had obliterated. There was no complication to the treatment and no rehemorrhage.
Rotational angiography has limitations, giving lower spatial resolution and little hemodynamic information, and its clinical role has to be defined. Nevertheless, some centers find it useful 33,34.
Indications for neurovascular radiosurgery
The major indications for neurovascular RS are BAVMs and DAVSs. Generally, indications can only be discussed in a management context, and management strategies may vary according to local expertise. Nevertheless, RS is most often superior to surgery for deep-seated lesions and those in eloquent cortical areas. The advantages of RS over embolization are that RS does not rely on vascular access and has a higher success rate as judged by complete nidus obliteration.
There are several subgroups among the cerebral arteriovenous shunts. One of those is Ren-du-Osler-Webers syndrome or hereditary hemorrhagic telangiectasias (HHT), in which 527% of the patients have cortical microfistulae or BAVMs, most often multiple 35. The risk of hemorrhage from a microfistulae or BAVM in HHT is probably smaller than in sporadic BAVMs 36.
In addition, BAVMs with very small volume seem as a rule to carry a low risk of hemorrhage 37. Conversely, the risk of hemorrhage in HHT patients may be higher than previously thought 38 and the decision to treat or not to treat is not clear-cut. However, if treated with RS microfistulae in HHT respond in a fashion similar to sporadic BAVMs 39.
Other BAVM subgroups are Wyburn-Masons syndrome, the cerebrofacial arteriovenous metameric syndrome with manifestations along the optic pathway, and proliferative angiopathy, an almost holohemispheric disease with limited arteriovenous shunting 40,41. Neither of these is an indication for treatment with RS.
The treatment of choice for vein of Galen arteriovenous malformations is embolization. RS has no role in the management of these patients 42,43.
Microsurgery and embolization are established treatment options for DAVS, and the indications and methods have recently been well described 44,45. However, a number case reports and single center series have shown that the efficacy of RS for DAVS is comparable to that of RS for BAVM 46-54.
Developmental venous anomalies (DVAs) are abnormalities in the brain venous system, previously named venous angiomas 55. They usually do not exhibit any arteriovenous shunts but may occasionally bleed, possibly from an associated cavernoma 56,57. The only reported series of RS for DVA showed a high incidence of complications 58. Being congenital variations in the normal venous system DVAs should not be treated with RS.
There is no agreement on whether RS has a role in the management of patients with cavernomas, regardless of whether they have bled or not. Some single center series suggest that prior hemorrhage from a cavernoma may be a risk factor for repeat hemorrhage and thus may warrant treatment with surgery or RS 59,60. With irradiation doses similar to those used in BAVM treatment the incidence of complications has been higher in cavernomas than in BAVMs, and it is unclear whether this treatment has reduced the risk of hemorrhage 61,62.
No treatment or surgery seems to be the most common management, but some advocate RS with low doses of radiation, claiming reduction of the risk of hemorrhage 62-66. In our institution patients with cavernomas are not treated with RS.
There are anecdotal reports on GKRS for arterial aneurysms. In view of the results of embolization and surgery there is no place for RS in the management of the patient with an aneurysm.
Target definition
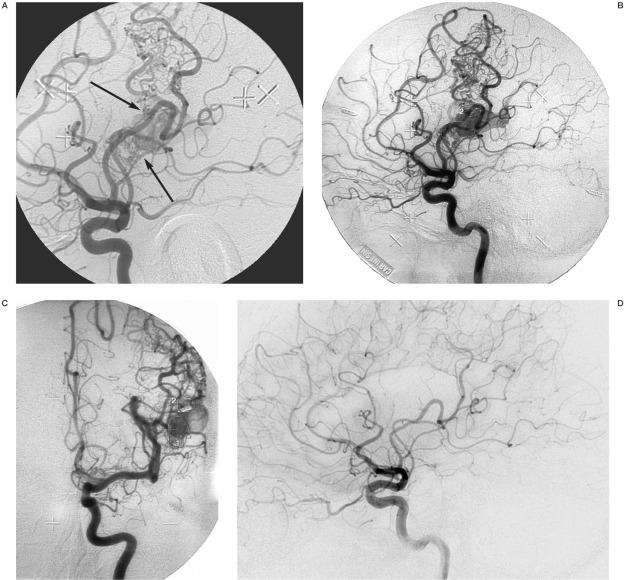
Variations in target definition practices are manifold and in the following discussion we rely to a large extent on our own practical experience. Target definition of BAVMs and DAVSs should ideally be based on biplane angiography with an image frame rate of no less than four per second. The BAVM nidus is delineated from the image or images when the draining veins begin to fill with contrast medium 29. Previously embolized lesions can have a very complex hemodynamic patterns that makes the delineation difficult (figure 2A-C). Because of the tendency of BAVMs to recruit transdural blood supply, in particular after partial embolization, the stereotactic angiography and follow-up studies should include injection into the external carotid artery 67. Very well circumscribed BAVMs with compact nidus can occasionally be better depicted by MRI or CT.
Figure 2.
Rolandic BAVM, previously embolized at another institution with occlusion of the Rolandic artery. The BAVM was supplied by an extensive pial arterial collateral system. A) Angiography with injection into the left internal carotid artery. Lateral view in the early phase of contrast media passage, just as the vein becomes visible. Two distinct shunting zones are evident (arrows) which were separately delineated before GKRS. B) Later phase of the angiogram, when the vein is filled with contrast medium. It is no longer possible to distinguish the shunts. The two separate target delineations are shown on this image. C) Posteroanterior view in a late phase of the angiogram with the two separate target delineations. Treatment was with 25Gy to the 50% isodose. D) After two years the BAVM was obliterated. There is no remaining arteriovenous shunt. Note that the pial arterial collateral network has regressed almost totally.
The intersection of two or more X-ray projections from angiography will define an "envelope" which is the maximum extension of the nidus, i.e in most cases an overestimation of the target volume 68. In a second step, the superimposition of the "envelope" onto axial images (MRI or CT) with consecutive editing of the volume allows for optimal nidus definition. MRI will also add data about, diffusion, perfusion and other tissue characteristics 69,70.
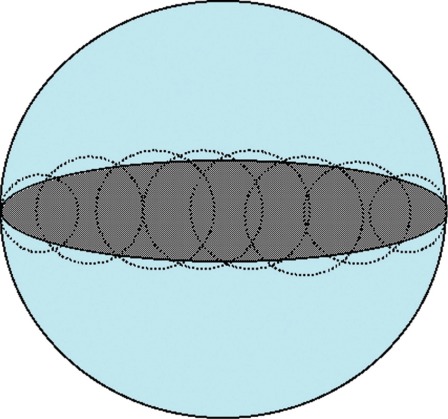
There are considerable interpersonal and intrapersonal variations in the results of target definition 34,71,72. Additionally, in some radiosurgical centers the nidus volume is defined by the nidus delineation, while in other centers it is defined in retrospect from the dose plan, i.e. as being the volume contained within the prescription isodose 73 In an irregular nidus the difference between the two methods of definition may be considerable. In addition, the conformity of the dose plan to the target may be good if the patient is treated with multiple isocenters, but less so if the patient is treated with only one spherical lesion (figure 3) Thus, the same target may be assigned different volume characteristics depending on the delineation, the dose planning and the irradiation equipment. For BAVMs, other measurements are also commonly used, such as the single largest diameter, corrected or uncorrected for the geometrical magnification 74,75.
Figure 3.
Two examples of 100% coverage of an ellipsoid volume. In the first case there is good conformity to the volume (grey ellipsoid) that has been covered with multiple lesions (dotted circles). In the second case the volume has been covered with only one single spherical lesion (black circle with blue background) as is common with less sophisticated or old LINACs. Both treatments will be with the same nominal minimum dose to the periphery of the target, but the amount of tissue irradiated and the dose distribution will differ significantly between the two "identical" treatments.
Unfortunately the differences in target definition, dose planning and volume calculation makes it almost impossible to compare treatment results between centers and even more so between modalities, unless all targets have been redefined according to the same protocol 76.
Radiobiological effects
Endothelial damage is the primary cause of the gradually developing occlusive effect that RS induces in the BAVM nidus, with minimal damage to the surrounding normal vasculature 77. The secondary proliferation of smoothmuscle cells and the elaboration of extracellular collagen by these cells, leads to progressive stenosis and obliteration of the BAVM nidus. The process may be identical in DAVSs, the nidus of which comprises thrombus, neovascularization and medial hyperplasia in basically normal vessels 78. The radiobiological mechanism behind the proposed effect of irradiation on cavernomas is unknown.
Outcome
In BAVM RS the rates of obliteration, complication and hemorrhage are interrelated and radiation-dose dependent. They are all accounted for in the available outcome models 24,79-82. Therefore, it does not provide the whole picture to discuss one outcome parameter without taking the others into account. Nevertheless, for didactic reasons the outcome parameters, i.e. obliteration, complication and hemorrhage, have been separated in the following discussion.
For DAVSs and cavernomas there are no outcome models.
Obliteration
The BAVM has been obliterated when the arteriovenous shunt has closed. MRI can give a very good indication of whether this is likely to have occurred, but to confirm cure catheter X-ray angiography is necessary 83,84.
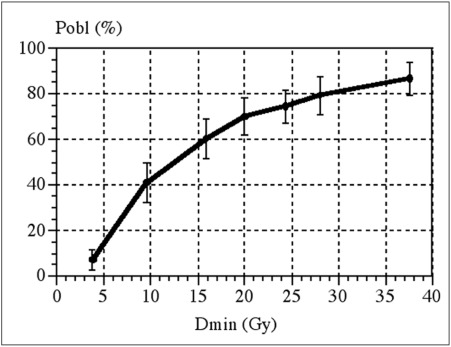
Some groups claim that the likelihood of obliteration depends only on the minimum radiation dose to the periphery of the BAVM, as shown in figure 4 80,85. If the dose plan conforms perfectly to the target volume the minimum isodose volume is the same as the prescription isodose volume. If the conformity is less good, the minimum dose to the target periphery will differ from the prescription isodose "envelope".
Figure 4.
The relation between minimum dose to the periphery of the BAVM and likelihood of obliteration over a 2-year period. Reproduced with permission from Ref. (80).
Other groups claim that there is a dose-volume effect, i.e. the larger the BAVM the less likely it is to be obliterated 86-88. However, in large BAVMs the dose to the periphery is usually reduced with the aim to decrease the risk of complications. Consequently, the apparent impact of volume on the likelihood of obliteration may result from difficulties in separating the two interdependent parameters, volume and radiation dose. In any case, the size of the BAVM affects the time period between RS and obliteration 89.
The gradually occlusive process usually begins after about six months or earlier and can continue up to five years after the treatment 90. Brain hemodynamics, metabolism and cognitive function normalizes with the regression of the arteriovenous shunt 70,91.
Lesions that respond to RS will either have shown significant flow reduction or have been obliterated after three years in most cases. The time lag until the shunt closes depends on the average radiation dose to the BAVM 73.
Thus, as the radiation dose to the BAVM periphery increases, so does the likelihood of obliteration and also the speed of the process.
Complications
In this context, adverse radiation effects or radiation-induced complications are the appearance of new or aggravated neurological symptoms together with imaging changes that are attributable to the irradiation (figure 5A-F) 79,92. Local damage to cranial nerves and nuclei may cause clinical consequences but not lead to changes visible on MRI images. Therefore such complications are not included in the present predictive model, and the likelihood of local damage should be added to the risk of complications expressed in figure 6. The transient edema that may appear around BAVMs, usually about a year after GKRS, is not considered a complication, unless it gives rise to symptoms 93.
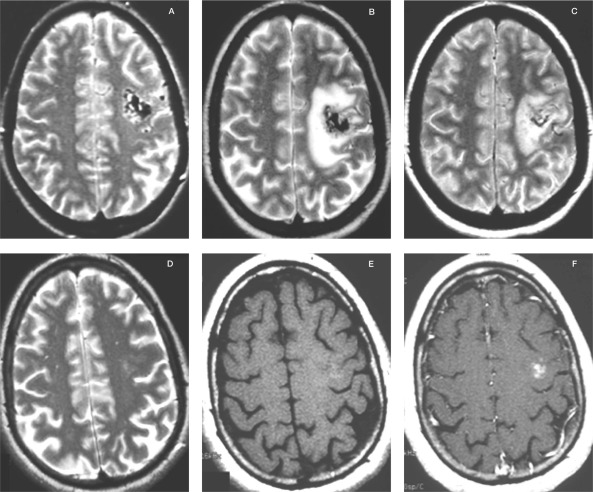
Figure 5.
MRI of a left frontal BAVM treated with a dose of 25Gy to the periphery in the GK. A) T2-weighted image at the time of the treatment. B) T2-weighted image eight months after the treatment. The patient developed seizures. C) T2-weighted image 12 months after the treatment. The patient was on steroids and anticonvulsive medication. D) T2-weighted image 24 months after the treatment. The patient was no longer on steroids or anticonvulsive drugs. E) T1-weighted image without Gadolinium 24 months after the treatment. F) T1-weighted image with Gadolinium 24 months after the treatment. Note the contrast enhancement at the position of the previously treated BAVM. The malformation was proven by angiography to be obliterated.
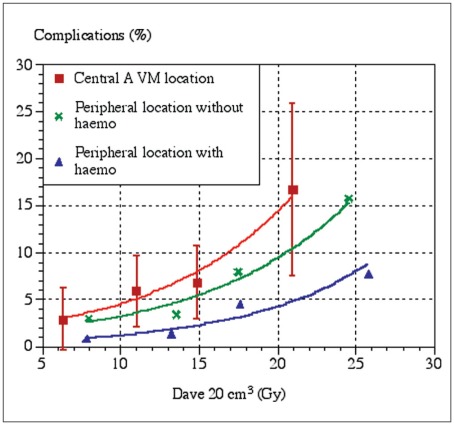
Figure 6.
The risk of radiation-induced complications as a function of the average dose in a 20cm3 volume containing the target and the surrounding tissues. Due to the small number of complications the confidence intervals are large. Reproduced with permission from Ref. (79).
The risk of developing complications after GKRS of a BAVM depends on the amount of energy imparted into the tissue and on the location of the malformation (figure 6). The risk seems to be higher for BAVMs with central location 79,94. It is not entirely clear whether this is because central structures are more prone to react to the radiation, or whether damage to structures in these locations more readily causes symptoms 95.
One study has shown that a prior hemorrhage decreased the risk of complications, at least in peripherally located BAVM (figure 6) 79. The mechanism is not clearly understood; however, it may be that the irradiated volumes due to the prior damage contained less viable tissue.
The higher the average dose of radiation and the larger the target volume, the higher the amount of energy imparted into the tissues will be, and consequently the risk of complications will increase proportionally 82. This has also been expressed as the "12Gy-volume" which is the volume of tissue that receives 12Gy or more of irradiation 96.
A transient complication usually lasts for three to nine months, occasionally longer. Symptoms can be alleviated by the administration of corticosteroids and sometimes antiepileptic drugs for a limited time. About 50% of the complications will leave some residual neurological deficit 97,98. At least for small BAVMs it seems that the risk of developing an adverse radiation effect is independent of which tissue is included in the target volume, i.e. BAVM or brain matter 82.
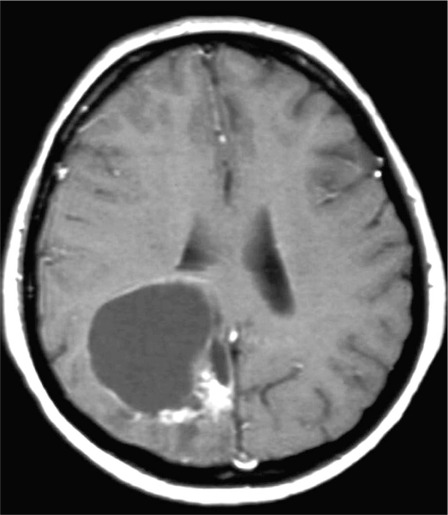
Long after the treatment, five years or more, pseudocysts can occasionally develop, and even bleed (figure 7) 99,100. These may have to be marsupialized or rarely extirpated.
Figure 7.
T1-weighted image with Gadolinium. Typical late post radiosurgery pseudocyst with an enhancing, more solid portion in the adjacent brain. The BAVM had been proven by angiography to be cured. The enhancing scar tissue was extirpated and the cyst was marsupialized. The patient recovered uneventfully.
Hemorrhage
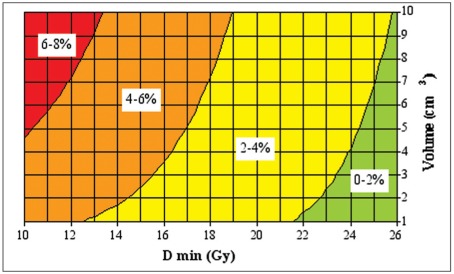
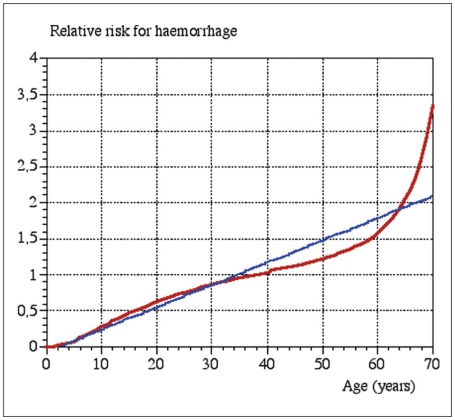
The risk of intracranial hemorrhage remains until the BAVM has been completely obliterated. At the time of treatment, it is obviously not possible to predict which malformation will obliterate and which will not. Data on hemorrhage during the latency period after the irradiation is therefore a mix of two cohorts, those whose BAVMs has been obliterated and therefore are not no longer at risk, and those with remaining BAVMs who are consequently still at risk. It seems that RS has a protective effect before obliteration but the data are somewhat contradictory 89,101,102. The risk of hemorrhage in the latency period depends on the minimum dose of radiation to the BAVM periphery as well as the BAVM volume and the age of the patient (figure 8,9) 81. A rough estimate is that the risk is between one third and half the risk of an untreated BAVM 81.
Figure 8.
The relation between the risk of hemorrhage during the two year latency period, BAVM volume and minimum radiation dose. Reproduced, with permission, from Ref. (81). The risk is also age-related. Therefore the calculated risk in the graph should be multiplied by the age-related relative risk factor from Fig. 9.
Figure 9.
The age-related risk factor for BAVM hemorrhage. Reproduced with permission from Ref. (81).
Occasionally a minute arteriovenous shunt will remain, without a visible nidus, i.e. its presence is revealed only by the premature contrast filling of a draining vein. We have never seen a hemorrhage from one of these remaining arteriovenous shunts, The only report in the literature of a bleed from such a persistent arteriovenous shunt later turned out to be one of the very rare occasions of BAVM growth adjacent to the obliterated original nidus 103,104.
The nidus angioarchitecure probably has an impact on the hemorrhage rate. However, little is known and there are only a few reports regarding this subject 102
In a patient with a DAVS, the risk for hemorrhage probably remains as long as there is cortical venous drainage from the shunt. So far the risk of a bleed from a DAVS during the period after RS before obliteration has not been very well quantified, but seems to be in the same range as for BAVMs 54.
Clinical outcome
Unless there is a complication, RS has little or no impact on the surrounding brain. Cognitive symptoms caused by the BAVM may improve and seizures usually decrease in frequency or cease completely as the BAVM regresses or is obliterated 91,105,106.
There is no evidence that RS for BAVMs in the pediatric population causes cognitive impairment, however data on children are very limited 107-111. In view of the possible long-term effects, caution is warranted.
Since the outcome of RS depends on the parameters discussed above, providing the crude complication rate is an inappropriate way to account for complications or hemorrhages. For example, in a patient with a history of hemorrhage, a peripherally located 1 ml BAVM irradiated with 25 Gy to the periphery would have roughly 75% probability of being obliterated within two years and around 85% within three years. The risk of complications (transient and permanent) would be about 1% and the risk of hemorrhage roughly 1% during the two-year period.
The corresponding figures for a peripherally located 3.3 ml BAVM that has not bled, irradiated with a minimum dose of 22Gy, would be a 67% probability of obliteration within two years, 5% risk of complications and 3% risk of hemorrhage.
Mortality associated with the treatment per se is virtually unheard of.
Retreatment
When a BAVM is treated for the second time the risk of adverse radiation reactions is higher than after the first treatment, all other factors being equal. The likelihood of obliteration is, however, the same as after the first treatment 24,112.
Permanency of obliteration
Recurrence of a previously treated BAVM or appearance of a new BAVM has occasionally been reported -almost exclusively in childrenafter RS with angiographically proven obliteration 103·113.
Hemorrhage despite obliteration but without recurrence has also been reported 101,114. The sporadic appearance does not permit quantification of the likelihood of recurrence. Most likely it is very unusual and therefore it seems unreasonable to propose a late follow-up angiography in patients with proven obliteration regardless of age. On the other hand, late control angiography in children with obliterated BAVMs has been proposed 111. Some centers follow pediatric patients with repeat MRI. However, since MRI has limited sensitivity for small BAVM a normal scan does not rule out the appearance of a new lesion 84.
Neuroradiology after RS
There are many post RS imaging protocols. In our institutions the routine follow-up protocol after GKRS of a BAVM is MRI with MRA one and two years after the treatment. If the BAVM is evident on the MRI two years after RS, a conventional angiography is performed. If the lesion is present at the two-year MRI we routinely perform an MRI and a conventional angiography three years after the treatment.
Common findings on the follow-up MRI are edema around the lesion after six to 18 months, which, however, often is asymptomatic and disappears before the two-year control (figure 4A-d) 93. When the BAVM is obliterated it may also leave a contrast enhancing "scar" that can be present for years (figure 4E,F). Signs of a significant adverse radiation reaction such as radionecrosis with tissue destruction and severe edema are unusual.
Several years after RS, fluid filled cavities or pseudocysts may develop in a low percentage of the patients (figure 6) 99,100.
BAVM-induced perfusion and diffusion changes in the brain have been shown to normalize with the obliteration process 70.
Management
Management strategies obviously differ according to local expertise and preferences. Additionally, in the absence of proper tools for an assessment of the risks and benefits of each treatment in each situation, management decisions tend to be subjective and, in addition, biased towards treatments available "in-house". Optimally, a BAVM management group should have all three treatment modalities available: RS as well as microneurosurgery and embolization. The treatment strategy should be tailored to each individual patient. In addition, no treatment at all may be the best option in some cases.
Large BAVMs are a management problem regardless of remedy and should in many instances probably not be treated at Al. 115,116. RS for large BAVMs should be conducted with caution until more data is published 89.
Consequently, these patients are best managed by a team that has long experience and expertise in all treatments.
Combined treatment
Most often the term combined treatment denotes either embolization followed by surgery, or embolization followed by RS. Published results are sometimes excellent 117. However, multimodality treatment should be used with caution, since the use of more than one treatment modality may add risk without adding benefit 118-120.
Future trends
In the modern RS equipment used in many centers today, dose planning software is already sophisticated and not much improvement can be expected. However, conformal irradiation to the target volume can be improved and the dose distribution optimized, which may improve outcome.
The development of outcome models for RS of large BAVMs (more than 10 ml) will help in the management of these difficult lesions.
Some work has been done on radiosensitizers in RS of BAVMs, but so far there has been no major breakthrough 121,122.
The merging of functional and anatomic imaging will become more useful, but this will probably benefit surgery and embolization more than RS. The role of magnetoencephalography in RS has not been defined.
Conclusions
Radiosurgery for BAVMs is an established treatment with clearly defined risks and benefits. The efficacy of radiosurgery for DAVS is probably similar but the treatment has not yet gained the same acceptance. Radiosurgical treatment of intracerebral cavernomas (cavernous hemangiomas) remains controversial.
Well founded predictive models for BAVM radiosurgery show:
• The probability of obliteration depends on the dose of radiation given to the periphery of the BAVM.
• The risk of adverse radiation effects depends on the total dose of radiation, i.e. the amount of energy imparted into the tissue. The risk is greater in centrally located lesions. The risk of damage to brainstem nucleii and cranial nerves must be added to the risk predicted by the model.
• The risk of hemorrhage during the time span before obliteration depends on the BAVM volume, the dose of radiation to the periphery of the lesion and the age of the patient. Central location is a probably also a risk factor.
References
- 1.Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001;95(4):633–637. doi: 10.3171/jns.2001.95.4.0633. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986;9(3):177–216. doi: 10.1007/BF01743136. [DOI] [PubMed] [Google Scholar]
- 3.Karhunen PJ, Penttila A, Erkinjuntti T. Arteriovenous malformation of the brain: imaging by postmortem angiography. Forensic Sci Int. 1990;48(1):9–19. doi: 10.1016/0379-0738(90)90267-3. [DOI] [PubMed] [Google Scholar]
- 4.Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations: Part I-Technique, morphology, and complications. Neurosurgery. 1996;39(3):448–457. doi: 10.1097/00006123-199609000-00004. discussion 457-459. [DOI] [PubMed] [Google Scholar]
- 5.Ondra SL, Troupp H, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow up assessment. Journal of Neurosurgery. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 6.Crawford P, West C, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49:1–10. doi: 10.1136/jnnp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman MF, Sciacca RR, et al. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47(2):389–396. doi: 10.1097/00006123-200008000-00023. discussion 397. [DOI] [PubMed] [Google Scholar]
- 8.Brown R, Wiebers D, et al. The natural history of unruptured intracranial arteriovenous malformations. Journal of Neurosurgery. 1988;68:352–357. doi: 10.3171/jns.1988.68.3.0352. [DOI] [PubMed] [Google Scholar]
- 9.ApSimon HT, Reef H, et al. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33(12):2794–2800. doi: 10.1161/01.str.0000043674.99741.9b. [DOI] [PubMed] [Google Scholar]
- 10.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93(5):1071–1078. doi: 10.1148/93.5.1071. [DOI] [PubMed] [Google Scholar]
- 11.Kjellberg RN, Hanamura T, et al. Bragg-peak protonbeam therapy for arteriovenous malformations of the brain. New England Journal of Medicine. 1983;309(5):269–274. doi: 10.1056/NEJM198308043090503. [DOI] [PubMed] [Google Scholar]
- 12.Barker FG, 2nd, Butler WE, et al. Dose-volume prediction of radiation-related complications after proton beam radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 2003;99(2):254–263. doi: 10.3171/jns.2003.99.2.0254. [DOI] [PubMed] [Google Scholar]
- 13.Solberg TD, Goetsch SJ, et al. Functional stereotactic radiosurgery involving a dedicated linear accelerator and gamma unit: a comparison study. J Neurosurg. 2004;101(Suppl 3):373–380. [PubMed] [Google Scholar]
- 14.Scheib SG, Gianolini S, et al. High precision radiosurgery and technical standards. Acta Neurochir Sup. 2004;91:9–23. doi: 10.1007/978-3-7091-0583-2_2. [DOI] [PubMed] [Google Scholar]
- 15.Leksell L. The stereotaxic method and radiosurgery to the brain. Acta Chirurgica Scandinavica. 1951;102:316–319. [PubMed] [Google Scholar]
- 16.Kondziolka D, Lunsford LD, et al. Radiosurgery and radiotherapy: observations and clarifications. J Neurosurg. 2004;101(4):585–589. doi: 10.3171/jns.2004.101.4.0585. [DOI] [PubMed] [Google Scholar]
- 17.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Sup 3):219–222. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 18.Pollock BE, Kline RW, et al. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 2000;48(3):817–824. doi: 10.1016/s0360-3016(00)00696-9. [DOI] [PubMed] [Google Scholar]
- 19.Sirin S, Kondziolka D, et al. Prospective staged volume radiosurgery for large arteriovenous malformations: indications and outcomes in otherwise untreatable patients. Neurosurgery. 2006;58(1):17–27. doi: 10.1227/01.neu.0000190653.42970.6b. discussion 17-27. [DOI] [PubMed] [Google Scholar]
- 20.Lindvall P, Wikholm G, et al. Combined effects of embolization and hypofractionated conformal stereotactic radiotherapy in arteriovenous malformations of the brain. Interventional Neuroradiology. 2005;11(3):223–228. doi: 10.1177/159101990501100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson B, Lax I, et al. Calculation of isoeffective doses and a/b value by comparing results following radiosurgery and radiotherapy for brain AVMs. J Neurosurg. 2006 doi: 10.3171/sup.2006.105.7.183. Accepted for publication (LKGS 2006 supplement) [DOI] [PubMed] [Google Scholar]
- 22.Kocher M, Wilms M, et al. Alpha/beta ratio for arteriovenous malformations estimated from obliteration rates after fractionated and single-dose irradiation. Radiother Oncol. 2004;71(1):109–114. doi: 10.1016/j.radonc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson B, Lindqvist M, et al. Long-term results after fractionated radiation therapy for large brain arteriovenous malformations. Neurosurgery. 2005;57(1):42–49. doi: 10.1227/01.neu.0000163095.56638.26. discussion 42-49. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson B, Kihlström L, et al. Gamma Knife surgery for previously irradiated arteriovenous malformations. Neurosurgery. 1998;42(1):1–6. doi: 10.1097/00006123-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Kondziolka D, Dempsey PK, et al. A comparison between magnetic resonance imaging and computed tomography for stereotactic coordinate determination. Neurosurgery. 1992;30(3):402–406. doi: 10.1227/00006123-199203000-00015. discussion 406-407. [DOI] [PubMed] [Google Scholar]
- 26.Mack A, Wolff R, et al. Analyzing 3-tesla magnetic resonance imaging units for implementation in radiosurgery. J Neurosurg. 2005;102(Sup):158–164. doi: 10.3171/jns.2005.102.s_supplement.0158. [DOI] [PubMed] [Google Scholar]
- 27.Novotny J, Jr, Vymazal J, et al. Does new magnetic resonance imaging technology provide better geometrical accuracy during stereotactic imaging? J Neurosurg. 2005;102(Sup):8–13. doi: 10.3171/jns.2005.102.s_supplement.0008. [DOI] [PubMed] [Google Scholar]
- 28.St George EJ, Butler P, Plowman PN. Can magnetic resonance imaging alone accurately define the arteriovenous nidus for gamma knife radiosurgery? J Neurosurg. 2002;97(5 Sup):464–470. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, Lindqvist M, et al. Stereotaxic angiography in gamma knife radiosurgery of intracranial arteriovenous malformations. American Journal of Neuroradiology. 1992;13:1107–1114. [PMC free article] [PubMed] [Google Scholar]
- 30.Guo WY, Nordell B, et al. Target delineation in radiosurgery for cerebral arteriovenous malformations. Assessment of the value of stereotaxic MR imaging and MR angiography. Acta Radiol. 1993;34(5):457–463. [PubMed] [Google Scholar]
- 31.Bednarz G, Downes B, et al. Combining stereotactic angiography and 3D time-of-flight magnetic resonance angiography in treatment planning for arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 2000;46(5):1149–1154. doi: 10.1016/s0360-3016(99)00530-1. [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Petrovich Z, et al. Study of magnetic resonance imaging-based arteriovenous malformation delineation without conventional angiography. Neurosurgery. 2004;54(5):1104. doi: 10.1227/01.neu.0000119327.53881.05. discussion 1108-1110. [DOI] [PubMed] [Google Scholar]
- 33.Colombo F, Cavedon C, et al. Three-dimensional angiography for radiosurgical treatment planning for arteriovenous malformations. J Neurosurg. 2003;98(3):536–543. doi: 10.3171/jns.2003.98.3.0536. [DOI] [PubMed] [Google Scholar]
- 34.Anxionnat R. Méthodes et outils pour le détourage des malformations artério-veineuses cérébrales dans un contexte multimodalité [Medical thesis] Nancy, France: Nancy: U.H.P.; 2003. [Google Scholar]
- 35.Fiorella ML, Ross DA, et al. Hereditary hemorrhagic telangiectasia: state of the art. Acta Otorhinolaryngol Ital. 2004;24(6):330–336. [PubMed] [Google Scholar]
- 36.Maher CO, Piepgras DG, et al. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32(4):877–882. doi: 10.1161/01.str.32.4.877. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson B, Lindquist C, et al. Annual risk for the first hemorrhage from untreated cerebral arteriovenous malformations. Minimally Invasive Neurosurgery. 1997;40(2):40–46. doi: 10.1055/s-2008-1053413. [DOI] [PubMed] [Google Scholar]
- 38.Easey AJ, Wallace GM, et al. Should asymptomatic patients with hereditary hemorrhagic telangiectasia (HHT) be screened for cerebral vascular malformations? Data from 22,061 years of HHT patient life. J Neurol Neurosurg Psychiatry. 2003;74(6):743–748. doi: 10.1136/jnnp.74.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maarouf M, Runge M, et al. Radiosurgery for cerebral arteriovenous malformations in hereditary hemorrhagic telangiectasia. Neurology. 2004;63(2):367–369. doi: 10.1212/01.wnl.0000130197.31844.16. [DOI] [PubMed] [Google Scholar]
- 40.Ducreux D, Meder JF, et al. MR perfusion imaging in proliferative angiopathy. Neuroradiology. 2004;46(2):105–112. doi: 10.1007/s00234-003-1045-6. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharya JJ, Luo CB, et al. Wyburn-Mason or Bonnet-Dechaume-Blanc as Cerebrofacial Arteriovenous Metameric Syndromes (CAMS): A new concept and a new classification. Interventional Neuroradiology. 2001;7(1):5–17. doi: 10.1177/159101990100700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watban JA, Rodesch G, et al. Transarterial embolization of vein of Galen aneurysmal malformation after unsuccessful stereotactic radiosurgery. Report of three cases. Childs Nerv Syst. 1995;11(7):406–408. doi: 10.1007/BF00717406. [DOI] [PubMed] [Google Scholar]
- 43.Lasjaunias P. Vascular Diseases in Neonates, Infants and Children. 1997 ed. Berlin: Springer-Verlag; 1997. [Google Scholar]
- 44.Sarma D, terBrugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol. 2003;46(3):206–220. doi: 10.1016/s0720-048x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 45.van Dijk JM, terBrugge KG, et al. Selective disconnection of cortical venous reflux as treatment for cranial dural arteriovenous fistulas. J Neurosurg. 2004;101(1):31–35. doi: 10.3171/jns.2004.101.1.0031. [DOI] [PubMed] [Google Scholar]
- 46.Barcia-Salorio JL, Herandez G, et al. Radiosurgical treatment of carotid-cavernous fistula. Appl Neurophysiol. 1982;45(4-5):520–522. doi: 10.1159/000101675. [DOI] [PubMed] [Google Scholar]
- 47.Barcia-Salorio JL, Soler F, et al. Stereotactic radiosurgery for the treatment of low-flow carotid-cavernous fistulae: results in a series of 25 cases. Stereotact Funct Neurosurg. 1994;63(1-4):266–270. doi: 10.1159/000100330. [DOI] [PubMed] [Google Scholar]
- 48.Guo WY, Pan DH, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. American Journal of Neuroradiology. 1998;19(6):1081–1087. [PMC free article] [PubMed] [Google Scholar]
- 49.Chandler HC, Jr, Friedman WA. Successful radiosurgical treatment of a dural arteriovenous malformation: case report. Neurosurgery. 1993;33(1):139–141. doi: 10.1227/00006123-199307000-00023. discussion 141-142. [DOI] [PubMed] [Google Scholar]
- 50.Pollock BE, Nichols DA, et al. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999;45(3):459–466. doi: 10.1097/00006123-199909000-00008. discussion 466-467. [DOI] [PubMed] [Google Scholar]
- 51.Shin M, Kurita H, et al. Stereotactic radiosurgery for tentorial dural arteriovenous fistulae draining into the vein of Galen: report of two cases. Neurosurgery. 2000;46(3):730–723. doi: 10.1097/00006123-200003000-00039. discussion 733-734. [DOI] [PubMed] [Google Scholar]
- 52.Friedman JA, Pollock BE, et al. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulas of the transverse and sigmoid sinuses. J Neurosurg. 2001;94(6):886–891. doi: 10.3171/jns.2001.94.6.0886. [DOI] [PubMed] [Google Scholar]
- 53.Pan DH, Chung WY, et al. Stereotactic radiosurgery for the treatment of dural arteriovenous fistulas involving the transverse-sigmoid sinus. J Neurosurg. 2002;96(5):823–829. doi: 10.3171/jns.2002.96.5.0823. [DOI] [PubMed] [Google Scholar]
- 54.Söderman M, Edner G, et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg. 2006;104(6):867–875. doi: 10.3171/jns.2006.104.6.867. [DOI] [PubMed] [Google Scholar]
- 55.Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1986;9(3):233–242. doi: 10.1007/BF01743138. [DOI] [PubMed] [Google Scholar]
- 56.Huber G, Henkes H, et al. Regional association of developmental venous anomalies with angiographically occult vascular malformations. Eur Radiol. 1996;6(1):30–37. doi: 10.1007/BF00619949. [DOI] [PubMed] [Google Scholar]
- 57.McLaughlin MR, Kondziolka D, et al. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998;43(2):195–200. doi: 10.1097/00006123-199808000-00001. discussion 200-201. [DOI] [PubMed] [Google Scholar]
- 58.Lindquist C, Guo WY, et al. Radiosurgery for venous angiomas [see comments] Journal of Neurosurgery. 1993;78(4):531–536. doi: 10.3171/jns.1993.78.4.0531. [DOI] [PubMed] [Google Scholar]
- 59.Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83(5):820–824. doi: 10.3171/jns.1995.83.5.0820. [DOI] [PubMed] [Google Scholar]
- 60.Aiba T, Tanaka R, et al. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83(1):56–59. doi: 10.3171/jns.1995.83.1.0056. [DOI] [PubMed] [Google Scholar]
- 61.Karlsson B, Kihlstrom L, et al. Radiosurgery for cavernous malformations. Journal of Neurosurgery. 1998;88(2):293–297. doi: 10.3171/jns.1998.88.2.0293. [DOI] [PubMed] [Google Scholar]
- 62.Pollock BE, Garces YI, et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93(6):987–991. doi: 10.3171/jns.2000.93.6.0987. [DOI] [PubMed] [Google Scholar]
- 63.Kondziolka D, Lunsford LD, et al. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83(5):825–831. doi: 10.3171/jns.1995.83.5.0825. [DOI] [PubMed] [Google Scholar]
- 64.Liu KD, Chung WY, et al. Gamma knife surgery for cavernous hemangiomas: an analysis of 125 patients. J Neurosurg. 2005;102(Sup):81–86. doi: 10.3171/jns.2005.102.s_supplement.0081. [DOI] [PubMed] [Google Scholar]
- 65.Liscak R, Vladyka V, et al. Gamma knife surgery of brain cavernous hemangiomas. J Neurosurg. 2005;102(Sup):207–213. doi: 10.3171/jns.2005.102.s_supplement.0207. [DOI] [PubMed] [Google Scholar]
- 66.Liscak R, Vladyka V, et al. Gamma knife radiosurgery of the brain stem cavernomas. Minim Invasive Neurosurg. 2000;43(4):201–207. doi: 10.1055/s-2000-11378. [DOI] [PubMed] [Google Scholar]
- 67.Söderman M, Rodesch G, Lasjaunias P. Transdural blood supply to cerebral arteriovenous malformations adjacent to the dura mater. Am J Neuroradiol. 2002;23(8):1295–1300. [PMC free article] [PubMed] [Google Scholar]
- 68.Bova FJ, Friedman WA. Stereotactic angiography: an inadequate database for radiosurgery? Int J Radiat Oncol Biol Phys. 1991;20(4):891–895. doi: 10.1016/0360-3016(91)90037-5. [DOI] [PubMed] [Google Scholar]
- 69.Petereit D, Mehta M, et al. Treatment of arteriovenous malformations with stereotactic radiosurgery employing both magnetic resonance angiography and standard angiography as a database. International Journal of Radiation Oncology Biology and Physics. 1993;25(2):309–313. doi: 10.1016/0360-3016(93)90353-w. [DOI] [PubMed] [Google Scholar]
- 70.Guo WY, Wu YT, et al. Toward normal perfusion after radiosurgery: perfusion MR Imaging with independent component analysis of brain arteriovenous malformations. American Journal of Neuroradiology. 2004;25(10):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 71.Söderman M. Volume determination and predictive models in the management of cerebral arteriovenous malformations [Medical thesis] Stockholm, Sweden: Karolinska Institute; 2000. [Google Scholar]
- 72.Buis DR, Lagerwaard FJ, et al. Stereotactic radiosurgery for brain AVMs: role of interobserver variation in target definition on digital subtraction angiography. Int J Radiat Oncol Biol Phys. 2005;62(1):246–252. doi: 10.1016/j.ijrobp.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 73.Karlsson B, Lindquist C, Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40(3):425–430. doi: 10.1097/00006123-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Drake CG. Cerebral arteriovenous malformations: considerations for and experience with surgical treatment in 166 cases. Journal of Neurosurgery. 1979;26:145–208. doi: 10.1093/neurosurgery/26.cn_suppl_1.145. [DOI] [PubMed] [Google Scholar]
- 75.Spetzler R, Martin N. A proposed grading system for arteriovenous malformations. Journal of Neurosurgery. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 76.Söderman M, Rodesch G, et al. Gamma knife outcome models as a reference standard in the embolisation of cerebral arteriovenous malformations. Acta Neurochir (Wien) 2001;143(8):801–810. doi: 10.1007/s007010170034. [DOI] [PubMed] [Google Scholar]
- 77.Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87(3):352–357. doi: 10.3171/jns.1997.87.3.0352. [DOI] [PubMed] [Google Scholar]
- 78.Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91(5):781–786. doi: 10.3171/jns.1999.91.5.0781. [DOI] [PubMed] [Google Scholar]
- 79.Karlsson B, Lax I, Soderman M. Factors influencing the risk for complications following Gamma Knife radiosurgery of cerebral arteriovenous malformations. Radiother Oncol. 1997;43(3):275–280. doi: 10.1016/s0167-8140(97)00060-1. [DOI] [PubMed] [Google Scholar]
- 80.Karlsson B, Lax I, Soderman M. Can the probability for obliteration after radiosurgery for arteriovenous malformations be accurately predicted? Int J Radiat Oncol Biol Phys. 1999;43(2):313–319. doi: 10.1016/s0360-3016(98)00396-4. [DOI] [PubMed] [Google Scholar]
- 81.Karlsson B, Lax I, Soderman M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49(4):1045–1051. doi: 10.1016/s0360-3016(00)01432-2. [DOI] [PubMed] [Google Scholar]
- 82.Lax I, Karlsson B. Prediction of complications in gamma knife radiosurgery of arteriovenous malformation [published erratum appears in Acta Oncol 1996; 35(3): 392] Acta Oncologica. 1996;35(1):49–55. doi: 10.3109/02841869609098479. [DOI] [PubMed] [Google Scholar]
- 83.Guo WY, Pan HC, et al. Do we need conventional angiography? The role of magnetic resonance imaging in verifying obliteration of arteriovenous malformations after Gamma Knife surgery. Stereotact Funct Neurosurg. 1996;66(Sup 1):71–84. doi: 10.1159/000099772. [DOI] [PubMed] [Google Scholar]
- 84.Fulbright RK, Chaloupka JC, et al. MR of hereditary hemorrhagic telangiectasia: prevalence and spectrum of cerebrovascular malformations. Am J Neuroradiol. 1998;19(3):477–484. [PMC free article] [PubMed] [Google Scholar]
- 85.Flickinger J, Pollock B, et al. A dose-response analysis of arteriovenous malformations obliteration after radiosurgery. International Journal of Radiation Oncology Biology and Physics. 1996;36(4):873–879. doi: 10.1016/s0360-3016(96)00316-1. [DOI] [PubMed] [Google Scholar]
- 86.Friedman W, Bova F, Mendenhall W. Linear accelerator radiosurgery for for arteriovenous malformations: the relationship of size to outcome. Journal of Neurosurgery. 1995;82:180–189. doi: 10.3171/jns.1995.82.2.0180. [DOI] [PubMed] [Google Scholar]
- 87.Meder J, Oppenheim C, et al. Cerebral arteriovenous malformations: The value of radiologic parameters in predicting response to radiosurgery. American Journal of Neuroradiology. 1997;18:1473–1483. [PMC free article] [PubMed] [Google Scholar]
- 88.Schwartz M, Sixel K, et al. Prediction of obliteration of arteriovenous malformations after radiosurgery: the obliteration prediction index. Canadian Journal of Neurological Sciences. 1997;24(2):106–109. doi: 10.1017/s0317167100021417. [DOI] [PubMed] [Google Scholar]
- 89.Pan DH, Guo WY, et al. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000;93(Sup 3):113–119. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 90.Guo WY, Pan DH, et al. Early irradiation effects observed on magnetic resonance imaging and angiography, and positron emission tomography for arteriovenous malformations treated by Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 1995;64(Sup 1):258–269. doi: 10.1159/000098787. [DOI] [PubMed] [Google Scholar]
- 91.Guo WY, Lee SM, et al. The Impact of Arteriovenous Malformation Radiosurgery on the Brain: From Morphology and Perfusion to Neurocognition. Stereotact Funct Neurosurg. 2006;84(4):162–169. doi: 10.1159/000094955. [DOI] [PubMed] [Google Scholar]
- 92.Plowman PN. Stereotactic radiosurgery. VIII. The classification of postradiation reactions. Br J Neurosurg. 1999;13(3):256–264. doi: 10.1080/02688699943655. [DOI] [PubMed] [Google Scholar]
- 93.Guo WY, Lindquist C, et al. Gamma knife surgery of cerebral arteriovenous malformations: serial MR imaging studies after radiosurgery. International Journal of Radiation Oncology Biology and Physics. 1993;25(2):315–323. doi: 10.1016/0360-3016(93)90354-x. [DOI] [PubMed] [Google Scholar]
- 94.Pollock BE, Gorman DA, Brown PD. Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg. 2004;100(2):210–214. doi: 10.3171/jns.2004.100.2.0210. [DOI] [PubMed] [Google Scholar]
- 95.Maruyama K, Kondziolka D, et al. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg. 2004;100(3):407–413. doi: 10.3171/jns.2004.100.3.0407. [DOI] [PubMed] [Google Scholar]
- 96.Flickinger JC, Kondziolka D, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. International Journal of Radiation Oncology Biology and Physics. 2000;46(5):1143–1148. doi: 10.1016/s0360-3016(99)00513-1. [DOI] [PubMed] [Google Scholar]
- 97.Guo W-Y. Radiological aspects of gamma knife radiosurgery for arteriovenous malformations and other non-tumoural disorders of the brain [Medical thesis] Stockholm, Sweden: Karolinska Institute; 1993. [PubMed] [Google Scholar]
- 98.Flickinger JC, Kondziolka D, et al. Complications from arteriovenous malformation radiosurgery: multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys. 1997;38(3):485–490. doi: 10.1016/s0360-3016(97)89481-3. [DOI] [PubMed] [Google Scholar]
- 99.Kihlstrom L, Guo WY, et al. Magnetic resonance imaging of obliterated arteriovenous malformations up to 23 years after radiosurgery [see comments] Journal of Neurosurgery. 1997;86(4):589–593. doi: 10.3171/jns.1997.86.4.0589. [DOI] [PubMed] [Google Scholar]
- 100.Pan HC, Sheehan J, et al. Late cyst formation following gamma knife surgery of arteriovenous malformations. J Neurosurg. 2005;102(Sup):124–127. doi: 10.3171/jns.2005.102.s_supplement.0124. [DOI] [PubMed] [Google Scholar]
- 101.Maruyama K, Kawahara N, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352(2):146–153. doi: 10.1056/NEJMoa040907. [DOI] [PubMed] [Google Scholar]
- 102.Nataf F, Ghossoub M, et al. Bleeding after radiosurgery for cerebral arteriovenous malformations. Neurosurgery. 2004;55(2):298–305. doi: 10.1227/01.neu.0000129473.52172.b5. discussion 305-306. [DOI] [PubMed] [Google Scholar]
- 103.Lindqvist M, Karlsson B, et al. Angiographic longterm follow-up data for arteriovenous malformations previously proven to be obliterated after gamma knife radiosurgery. Neurosurgery. 2000;46(4):803–808. doi: 10.1097/00006123-200004000-00006. discussion 809-810. [DOI] [PubMed] [Google Scholar]
- 104.Guo WY, Karlsson B, et al. Even the smallest remnant of an AVM constitutes a risk of further bleeding. Case report. Acta Neurochirurgica. 1993;121(3-4):212–215. doi: 10.1007/BF01809278. [DOI] [PubMed] [Google Scholar]
- 105.Steiner L, Lindquist C, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J. Neurosurgery. 1992;77:1–8. doi: 10.3171/jns.1992.77.1.0001. [DOI] [PubMed] [Google Scholar]
- 106.Schauble B, Cascino GD, et al. Seizure outcomes after stereotactic radiosurgery for cerebral arteriovenous malformations. Neurology. 2004;63(4):683–687. doi: 10.1212/01.wnl.0000134659.95217.6e. [DOI] [PubMed] [Google Scholar]
- 107.Levy EI, Niranjan A, et al. Radiosurgery for childhood intracranial arteriovenous malformations. Neurosurgery. 2000;47(4):834–841. doi: 10.1097/00006123-200010000-00008. discussion 841-842. [DOI] [PubMed] [Google Scholar]
- 108.Eder HG, Leber KA, et al. The role of gamma knife radiosurgery in children. Childs Nerv Syst. 2001;17(6):341–346. doi: 10.1007/s003810000435. discussion 347. [DOI] [PubMed] [Google Scholar]
- 109.Smyth MD, Sneed PK, et al. Stereotactic radiosurgery for pediatric intracranial arteriovenous malformations: the University of California at San Francisco experience. J Neurosurg. 2002;97(1):48–55. doi: 10.3171/jns.2002.97.1.0048. [DOI] [PubMed] [Google Scholar]
- 110.Nataf F, Schlienger M, et al. Radiosurgery of cerebral arteriovenous malformations in children: a series of 57 cases. International Journal of Radiation Oncology Biology and Physics. 2003;57(1):184–195. doi: 10.1016/s0360-3016(03)00445-0. [DOI] [PubMed] [Google Scholar]
- 111.Nicolato A, Lupidi F, et al. Gamma knife radiosurgery for cerebral arteriovenous malformations in children/adolescents and adults. Part I: Differences in epidemiologic, morphologic, and clinical characteristics, permanent complications, and bleeding in the latency period. Int J Radiat Oncol Biol Phys. 2006;64(3):904–913. doi: 10.1016/j.ijrobp.2005.07.983. [DOI] [PubMed] [Google Scholar]
- 112.Pollock BE, Kondziolka D, et al. Repeat stereotactic radiosurgery of arteriovenous malformations: factors associated with incomplete obliteration. Neurosurgery. 1996;38(2):318–324. doi: 10.1097/00006123-199602000-00016. [DOI] [PubMed] [Google Scholar]
- 113.Kader A, Goodrich J, et al. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. Journal of Neurosurgery. 1996;85:14–18. doi: 10.3171/jns.1996.85.1.0014. [DOI] [PubMed] [Google Scholar]
- 114.Shin M, Kawahara N, et al. Risk of hemorrhage from an arteriovenous malformation confirmed to have been obliterated on angiography after stereotactic radiosurgery. J Neurosurg. 2005;102(5):842–846. doi: 10.3171/jns.2005.102.5.0842. [DOI] [PubMed] [Google Scholar]
- 115.Wikholm G, Lundqvist C, Svendsen P. The Goteborg cohort of embolized cerebral arteriovenous malformations: a 6-year follow-up. Neurosurgery. 2001;49(4):799–805. doi: 10.1097/00006123-200110000-00004. discussion 805-806. [DOI] [PubMed] [Google Scholar]
- 116.Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98(1):3–7. doi: 10.3171/jns.2003.98.1.0003. [DOI] [PubMed] [Google Scholar]
- 117.Spetzler RF, Martin NA, et al. Surgical management of large AVM's by staged embolization and operative excision. J Neurosurg. 1987;67(1):17–28. doi: 10.3171/jns.1987.67.1.0017. [DOI] [PubMed] [Google Scholar]
- 118.Nakstad PH, Nornes H. Superselective angiography, embolisation and surgery in treatment of arteriovenous malformations of the brain. Neuroradiology. 1994;36(5):410–413. doi: 10.1007/BF00612131. [DOI] [PubMed] [Google Scholar]
- 119.Söderman M, Andersson T, et al. Management of patients with brain arteriovenous malformations. Eur J Radiol. 2003;46(3):195–205. doi: 10.1016/s0720-048x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 120.Guo WY, Wikholm G, Karlsson B, et al. Combined embolization and Gamma Knife radiosurgery for cerebral arteriovenous malformations. Acta Radiologica. 1993;34(6):600–606. [PubMed] [Google Scholar]
- 121.Major O, Szeifert GT, et al. Effect of a single highdose gamma irradiation on cultured cells in human cerebral arteriovenous malformation. J Neurosurg. 2002;97(5 Sup):459–463. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 122.Sims EC, Plowman PN. Stereotactic radiosurgery XII. Large AVM and the failure of the radiation response modifier gamma linolenic acid to improve the therapeutic ratio. British Journal of Neurosurgery. 2001;15(1):2834. doi: 10.1080/026886901300004058. [DOI] [PubMed] [Google Scholar]