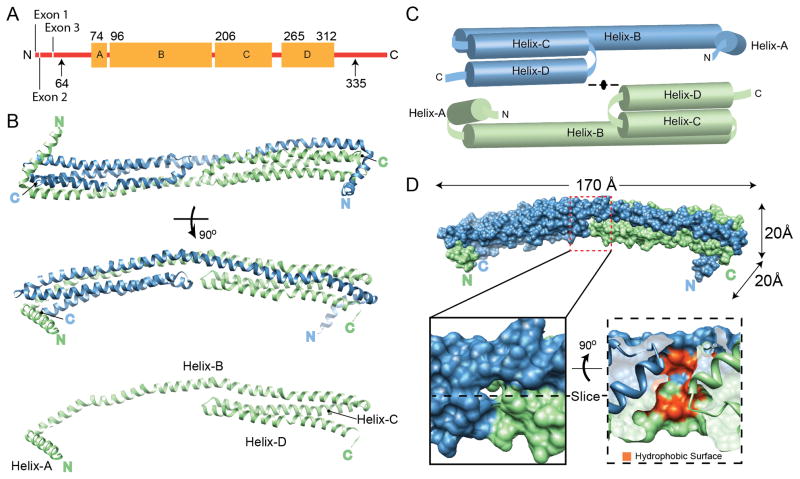
Figure 1.
Structure of apoA-IV64-335. (A) Linear diagram of human apoA-IV. Arrows indicate the extent of the protein used for crystallization (64-335). The residues that were visible in the structure (74-312) are shown with the four major helical domains (A–D) indicated as orange boxes. (B) The structure, rotated through 90°, is depicted as blue and green ribbons interacting through a helical domain swap mechanism. The green monomer is also shown in the absence of the blue monomer. (C) Schematic representation of the dimer pulled apart. (D) Space filling representation of the dimer with molecular dimensions indicated. A cavity at the center of the structure is highlighted, showing the exposure of hydrophobic core residues in red (see also Figure S1). See also Figure S8.