Figure 2.
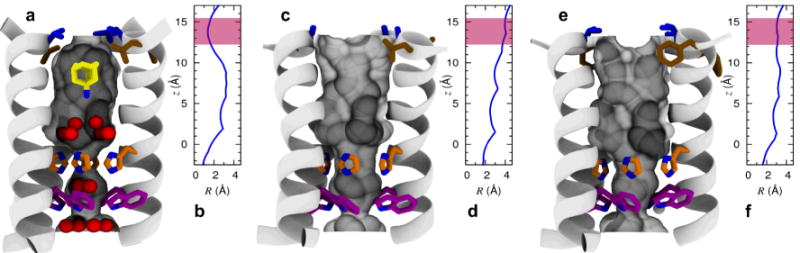
Shape modulation of the drug-binding pocket in the pore of A/M2 channel. A cartoon representation of the A/M2-TM helix bundle is shown for WT (a,b), V27A (c,d), and L26F (e,f). The molecular surface of the channel pore is highlighted and pore-lining residues Leu/Phe26 (brown), Val/Ala27 (blue), His37 (orange), and Trp41 (violet) are shown as sticks. Pore water molecules and amantadine are shown for wt as red spheres and sticks, respectively. The positions of water oxygens and amantadine are obtained from the crystal structure28 (PDB: 3LBW), and the ssNMR structure of the A/M2-amantadine complex29 (PDB: 2KQT), respectively. For each structure, the radius of the pore (computed using Hole47) is plotted as a function of the displacement along the channel axis in the region between His37 and Val-Ala27; the range of values corresponding to the entry region of the channel pore is highlighted in pink.
