Abstract
The Mammary Prevention 3 (MAP.3) placebo-controlled randomized trial in 4,560 high-risk postmenopausal women showed a 65% reduction in invasive breast cancer with the use of exemestane at 35 months median follow-up. Few differences in adverse events were observed between the arms, suggesting a promising risk:benefit balance with exemestane for use in chemoprevention. Yet, the MAP.3 design and implementation raise concerns regarding limited data maturity and not prospectively including key bone-related and other toxicities as study end points. Exemestane for prevention is juxtaposed against selective estrogen receptor modulators and the other aromatase inhibitors. Additional issues for prevention, including the influence of obesity, alternative dosing, and biomarker use in phase III trials, are addressed.
Significance
The recently completed MAP.3 trial of exemestane for breast cancer prevention offers a potential new standard for pharmaceutical risk reduction in high-risk postmenopausal women. In addition to describing key findings from the publication of MAP.3 and related trials, our review undertakes a detailed analysis of the strengths and weaknesses of MAP.3 as well as the implications for future prevention research.
Introduction
Aromatase inhibitors and exemestane
Overall, $75% of breast cancers are estrogen receptor (ER) positive, with the percentage increasing with age (1). These ER-positive breast cancers are presumed to be dependent on estrogen for stimulation of cell proliferation. Although there is a better prognosis with ER-positive compared with ER-negative tumors, the frequency with which ER-positive tumors occurs is considerably higher, making them responsible for most breast cancer deaths. In contrast to selective estrogen receptor modulators (SERM), which interfere with estrogen action, aromatase inhibitors (AI) inhibit estrogen synthesis and offer an alternative approach to the treatment and potential prevention of ER-positive breast cancers. Two SERMs, tamoxifen and raloxifene, are both approved in the United States for breast cancer risk reduction in women at increased risk, with raloxifene approval being restricted to postmenopausal women. Two additional SERMs, lasofoxifene and arzoxifene, have been shown in phase III trials to reduce the incidence of breast cancer (2 – 4). Despite their approval, the toxicities of tamoxifen and raloxifene as perceived by patients and their primary care physicians have interfered with their acceptance in the community by healthy women who might benefit from their risk-reducing properties. Furthermore, the preventive benefits of these two SERMs do not extend to all breast cancers, with at least 50% of ER-positive and 100% of ER-negative cancers not benefiting from these estrogen-targeting agents. The need for drugs with the ability to reduce the risk of a larger spectrum of breast cancers, together with the concern about the undesirable risk:benefit balance of tamoxifen and raloxifene, has prompted a search for alternative pharmaceutical approaches to prevent this disease. Given this backdrop, the emerging evidence for preventive properties of AIs in the form of reduced primary contralateral breast cancers in adjuvant trials has stimulated interest in testing each of the latter agents for primary breast cancer prevention in phase III trials. In contrast to the increased risk of endometrial cancer (tamoxifen) and thromboembolic disease (both SERMs, with less risk with raloxifene than tamoxifen), AIs were anticipated and ultimately shown to elicit adverse effects related to estrogen deficiency, particularly thinning of bone and consequential fractures.
All three third-generation AIs—anastrozole, letrozole and exemestane—are extremely potent in inhibiting conversion of androgens to estrogens by aromatase; at clinical doses all three inhibit the enzyme by more than 97% (5). Despite subtle differences in activity (5, 6), the three AIs exhibit comparable efficacy in the clinical setting (7). Exemestane, originally developed in Milan, Italy, by Farmitalia Carlo Erba as FCE 24304 (8), differs from the other two third generation AIs; it has a steroidal structure (Fig. 1) and does not impair aromatase's production of estrogen via a competitive inhibition mechanism but, rather, binds irreversibly to the enzyme (8). The steroidal nature of exemestane suggested that it might behave like a weak androgen in bone so as to counteract the resorptive effect of estrogen depletion (9). The expected result would thus be less bone toxicity than seen with anastrozole and letrozole. Preclinical data from ovariectomized rats administered exemestane showed improvements in several biomarkers of bone strength [bone mineral density (BMD), bone histomorphometry, and bone resorption markers] (9, 10). Although promising as the single third-generation AI to avoid major bone toxicity, the animal findings have not been validated in clinical trial in humans, with all three third-generation AIs displaying comparable toxicity in bone. Following 2 years of treatment with exemestane, postmenopausal women with early breast cancer showed a modest increase in bone loss from the femoral neck, but not from the lumbar spine (11). Exemestane administered for 1 year to healthy postmenopausal women resulted in reversible bone resorption, as indicated by increases in N-telopeptide, but without comparable changes in bone-specific alkaline phosphatase or BMD (12).
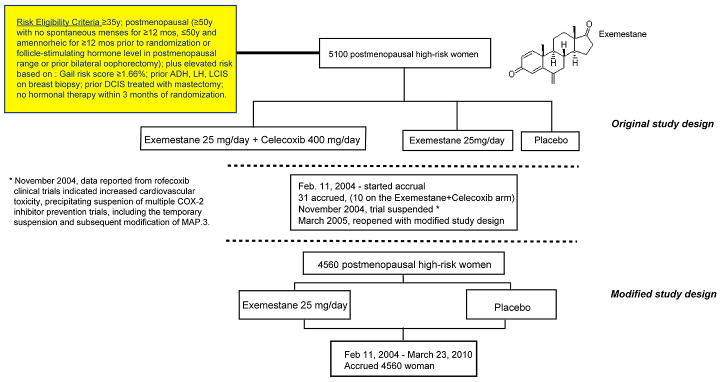
Figure 1. Mammary Prevention 3 (MAP.3) Trial Schema.
Adjuvant Trial Data with AIs
The three AIs have been tested in definitive phase III adjuvant trials, each of which used a different study design (13). The value of these adjuvant trials for prevention is in their inclusion of new primary breast cancers in the contralateral breast as secondary end points (Fig. 2). Individual adjuvant trials and the results of a meta-analysis are included in Figure 2. Criteria for inclusion of an article in the meta-analysis were as follows: (i) it had to be the principal and most updated published report of a clinical trial (highest mean/median follow-up time) evaluating treatment with AIs as adjuvant treatment in postmenopausal women with early breast cancer; (ii) it had to be independent from other reports to avoid giving double weight to estimates derived from the same study; (iii) it had to have sufficient information to allow adequate estimation of the HR and/or OR and 95% CIs (i.e., crude data: number of events by arms or adjusted estimates and SEM, CIs, or P values) to estimate contralateral breast cancer risk under treatment with AIs compared with any other treatment or placebo. A total of 14 reports on 10 clinical trials (refs. 14–23; Fig. 2) were retrieved and checked for relevance in terms of intervention, population studied, and reporting of contralateral breast cancer incidence data. The TEAM (22) study was not included because contralateral cancer incidence data were not available. Data on the ITA study were retrieved from the first study report published (21) because in the most recent article (23) information on contralateral breast cancer events per arm was not available. Because contralateral breast cancer events in the ABCSG-8 study were not retrievable in the literature but only in combination with the very similar ARNO study in the report by Jakesz et al. (18), results from these two studies were considered in combination in a single cancer risk estimate.
Figure 2.
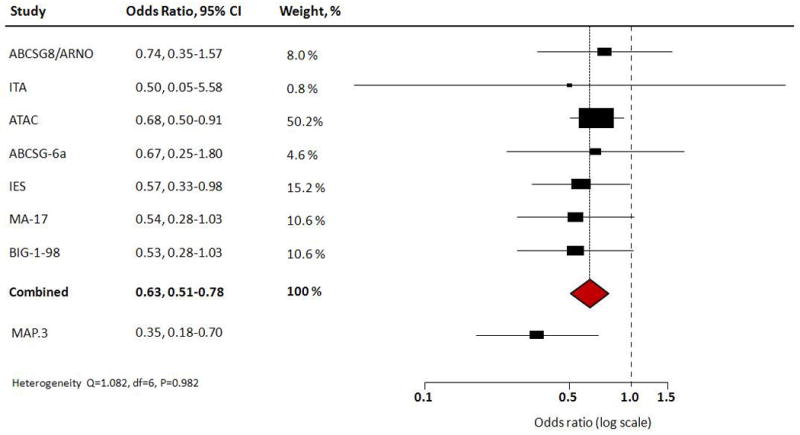
Forest plots of new primary breast cancer events in contralateral breast in adjuvant trials of AIs. A total of 14 reports on 10 clinical trials (14–23) [Austrian Breast Cancer Study Group (ABCSG)-6a; Arimidex-Nolvadex (ARNO); ARNO95; Italian Tamoxifen Anastrozole (ITA); Arimidex, Tamoxifen Alone or in Combination (ATAC); ABCSG-6; Tamoxifen Exemestane Adjuvant Multinational (TEAM); Intergroup Exemestane Study (IES); NCI Canada Mammary (MA)-17; Breast International Group (BIG) 1-98] were included in the analyses used to generate this figure (see text for statistical methods).
For each of the clinical trials selected, we extracted HRs and their 95% CIs from the published articles, or, when a time-to-event risk estimate was not available, we calculated ORs by considering the number of contralateral breast cancer events per arm. Association between AIs and contralateral breast cancer incidence across selected studies was computed as a combined OR with 95% CI, pooling the study-specific estimates (HRs or ORs) using randomeffects models fitted by using SAS (Proc Mixed) with maximum likelihood estimates.
The Arimidex (Anastrozole), Tamoxifen Alone or in Combination (ATAC) trial showed at a 100-month median follow-up a significant 40% reduction (HR = 0.60; 95% CI, 0.42–0.85) in contralateral breast cancers with anastrozole compared with tamoxifen (24). Letrozole was examined in the adjuvant setting in the Breast International Group (BIG) 1-98 trial comparing the AI to tamoxifen directly or in sequence in a four-arm trial (14, 25). Again, contralateral breast cancers were less frequent with letrozole than tamoxifen (0.6% of women administered letrozole vs. 1.1% of women administered tamoxifen) at a median followup of 51 months (14). In the National Cancer Institute of Canada (NCIC) Mammary (MA)-17 trial for earlystage breast cancer, a different study design randomizing women to extended adjuvant treatment with letrozole versus placebo following 5 years of tamoxifen treatment also supported the benefit of the AI by showing a relative reduction of 46% in contralateral breast cancers after a median of 2.4 years of letrozole treatment (15). Similarly, at a median follow-up of 55.7 months the Intergroup Exemestane Study (IES) showed that contralateral breast cancers were significantly reduced when treatment was switched to exemestane after 2 to 3 years of tamoxifen compared with a full 5 years of tamoxifen, with an HR of 0.57 (95% CI, 0.33–0.98; P = 0.04) (16). Other trials using these AIs confirm the benefit of the AI relative to the SERM comparator arm with respect to contralateral breast cancer risk reduction. A meta-analysis of 9,000 patients participating in all available trials of AIs versus tamoxifen indicated a 35% reduction in contralateral breast cancer in the arm involving a switch to an AI after 2 to 3 years of tamoxifen treatment compared with the arm treated with tamoxifen for the full course of the trial (26).
Toxicity as a result of exemestane treatment in the adjuvant setting primarily involves bone and musculoskeletal events. An analysis of a 206-participant subgroup in the IES trial reported at a median follow-up of 58 months revealed a rapid decline in BMD from baseline during the first 6 months following the switch from tamoxifen to exemestane: 0.051 g/cm3 (2.7%; 95% CI, 2.0–3.4; P < 0.0001) at the lumbar spine and 0.025 g/cm3 (1.4%; 95% CI, 0.8–1.9; P < 0.0001) at the total hip (27). This finding contrasts with the finding of the tamoxifen-only arm, in which no statistically significant changes in BMD were observed. At a 55.7-month median follow-up, with a median exposure to exemestane of 30 months, the parent IES trial results showed a total of 277 patients with fractures, 162 (7%) and 115 (5%) patients in the exemestane and tamoxifen arms, respectively (OR = 1.45; 95% CI, 1.13-1.87; P = 0.003) (16, 27). A significant difference was also seen between the arms in the IES parent study in numbers (percentage) of patients with musculoskeletal symptoms, including arthritis [405 (17.5%) vs. 341 (14.6%); P = 0.008], arthralgia [483 (20.8%) vs. 354 (15.1%); P < 0.0001], and musculoskeletal pain [596 (25.7%) vs. 474 (20.3%); P < 0.0001], for exemestane compared with tamoxifen (16).
NCIC Mammary Prevention 3 Trial
The reduction in contralateral breast cancer as a secondary end point in the AI adjuvant trials strongly suggested that AIs have potential to prevent breast cancer in healthy high-risk women, possibly to an even greater extent than SERMs. Inspired by such contralateral breast cancer findings in the IES trial, the developers of the Mammary Prevention 3 (MAP.3) trial designed it to test the hypothesis that exemestane would decrease the incidence of invasive breast cancer in high-risk women. To be eligible, a woman had to be postmenopausal, have a Gail risk score ≥1.66% for developing breast cancer within 5 years, or have a prior history of atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), or prior ductal carcinoma in situ (DCIS) treated with mastectomy (Fig. 1).
Design and Implementation
The initial plan for MAP.3 was to randomize 5,100 increased-risk postmenopausal women to 25 mg/d of exemestane + 400 mg/d of celecoxib versus 25 mg/d of exemestane versus placebo for a total of 5 years (28) (Fig. 1). The rationale for including celecoxib was prior observations of overexpression of cyclooxygenase-2 (COX-2) in both premalignant and invasive breast cancer lesions, together with epidemiologic data suggesting a reduction in breast cancer in women treated with COX-2 inhibitors (29, 30). Furthermore, at the time a widespread view existed that celecoxib was safe and well tolerated. Following activation of MAP.3, 31 participants were recruited between September and November 2004. However, in September 2004 rofecoxib (Vioxx; Merck), in the Adenomatous Polyp Prevention on VIOXX (APPROVe) trial (31), and in late December 2004 celecoxib, in the Adenoma Prevention with Celecoxib (APC) trial (32), were both observed to be associated with increased risk of cardiovascular disease. Based on these findings, the MAP.3 trial was temporarily suspended and its design modified to omit the celecoxib-containing arm and randomize 4,560 postmenopausal women in a 1:1 ratio to exemestane or placebo. Following the trial's reopening in March 2005, 4,560 eligible women were accepted through March 23, 2010, with the protocol-targeted event rate achieved on November 5, 2010.
Results
Key baseline characteristics, including median age, breast cancer risk factors and risk level, prior therapy with hormonal and bone drugs, and BMD, were equally distributed between the exemestane and placebo arms (Table 1) (33). Regarding the primary endpoint, 43 invasive breast cancers were observed, with a cumulative incidence of 11 in the exemestane arm and 32 in the placebo arm, reflecting an annual incidence of 0.19% and 0.55%, respectively, and an HR of 0.35 (95% CI, 0.18–0.70; P = 0.002). The benefit was seen only for ER-positive tumors: 7 (0.12%/year) with exemestane and 27 (0.46%/year) with placebo, with an HR of 0.27 (95% CI, 0.14–0.60; P < 0.001). Importantly, ERnegative tumors showed no benefit or adverse effect from exemestane compared with placebo (HR = 0.80; 95% CI, 0.21–2.98; P = 0.74). Invasive tumors combined with DCIS also showed a significant reduction with exemestane versus placebo (HR = 0.47; 95% CI, 0.27–0.79; P = 0.004), but the incidence of DCIS alone was not reduced with exemestane relative to placebo (HR = 0.65; 95% CI, 0.28–1.51; P = 0.31). Similarly, the combined category of intraepithelial neoplasia (IEN) subtypes including ADH, ALH, and LCIS was not significantly reduced with exemestane (HR = 0.36; 95% CI, 0.11–1.12; P = 0.08). To prevent one case of invasive breast cancer, the number needed to treat (NNT), a widely used measure of treatment benefit (34), with exemestane was 94 over 3 years and projected to be 26 over 5 years. These projections must be viewed with caution, however, given the fact that MAP.3 included few women who completed a full 5 years of therapy and the median follow-up was considerably less than 5 years.
Table 1. Baseline variables and outcomes in MAP.3.
| Variable | Exemestane | Placebo | HR (95% CI) | P value |
|---|---|---|---|---|
| Baseline | ||||
| Number of women | 2,285 | 2,275 | NA | NA |
| Median age (interquartile range), y | 62.5 (38.5–88.2) | 62.4 (37.1–89.9) | NA | NA |
| Breast cancer risk factors | ||||
| 5-year Gail risk >1.66%: n (%) | 929 (40.7) | 905 (39.8) | NA | NA |
| Age ≥60 years: n (%) | 1,114 (48.8) | 1,126 (49.5) | NA | NA |
| Prior history of IEN: n (%) | ||||
| ADH, ALH, LCIS pooled | 185 (8.1) | 188 (8.3) | NA | NA |
| DCIS- treated with mastectomy | 56 (2.5) | 56 (2.5) | NA | NA |
| Bone variables | ||||
| Prior bisphosphonate use: n (%) | 427 (18.7) | 414 (18.2) | NA | NA |
| Current osteoporosis at baseline: n (%) | 303 (13.3) | 293 (12.9) | NA | NA |
| History of clinical fractures: n (%) | 409 (17.9) | 400 (17.6) | NA | NA |
| Outcomes, primary endpoint: n [annual incidence (%)] | ||||
| Invasive breast cancer | 11 (0.19) | 32 (0.55) | (0.18–0.70) | 0.002 |
| Outcomes, secondary endpoints: n [annual incidence (%)] | ||||
| Breast cancer endpoints | ||||
| Invasive BC: ER positive | 7 (0.12) | 27 (0.46) | 0.27 (0.12–0.60) | <0.001 |
| Invasive BC: ER negative | 4 (0.07) | 5 (0.09) | 0.80 (0.21–2.98) | 0.74 |
| DCIS | 9 (0.16) | 14 (0.24) | 0.65 (0.28–1.51) | 0.31 |
| Combined invasive BC + DCIS | 20 (0.35) | 44 (0.77) | 0.47 (0.27–0.79) | 0.004 |
| Combined IEN: ADH, ALH, LCIS | 4 (0.07) | 11 (0.20) | 0.36 (0.11–1.12) | 0.08 |
| Adverse events (assessed in n women) | n = 2,240 | n = 2,248 | ||
| Musculoskeletal | ||||
| Bone: clinical fractures | 149 (6.7) | 143 (6.4) | NA | 0.72 |
| Bone: new osteoporosis | 37 (1.7) | 30 (1.3) | NA | 0.39 |
| Musculoskeletal: arthritis | 247 (11) | 196 (9) | NA | 0.01 |
| Arthralgia | 665 (30) | 606 (27) | NA | 0.04 |
| Myalgia | 147 (7) | 192 (9) | NA | 0.01 |
| Gastrointestinal events | ||||
| Diarrhea | 118 (5) | 75 (3) | NA | 0.002 |
| Nausea | 155 (7) | 122 (5) | NA | 0.04 |
| Cardiovascular | 106 (4.7) | 111 (4.9) | NA | 0.78 |
| Endocrine: hot flashes | 900 (40) | 718 (32) | NA | <0.001 |
| Sexual function: vaginal dryness | 352 (16) | 343 (15) | NA | 0.68 |
| Other cancers | 43 (1.9) | 38 (1.7) | NA | 0.58 |
| SF-36 and MENQOL | ||||
| SF-36: bodily pain | NA | NA | NA | <0.001 |
| MENQOL: vasomotor | NA | NA | NA | <0.001 |
| MENQOL: sexual | NA | NA | NA | 0.01 |
NOTE: Values are from Goss et al. (33) and accompanying Supplementary Materials at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1103507/suppl_file/nejmoa1103507_appendix.pdf
Abbreviations: BC, breast cancer; CI, confidence interval; IEN, intraepithelial neoplasia; NA, not applicable.
Adverse symptoms and events were noted in 88% of women treated with exemestane and 85% of women taking placebo, reflecting a significant difference between the groups (P = 0.003). Musculoskeletal events and menopausal symptoms were prominent among the observed toxicities. Arthritis was observed in 11% of women in the exemestane group and 9% in the placebo group (P = 0.01), joint pain in 30% in the exemestane group versus 27% in the placebo group (P = 0.04), and muscle pain in 7% in the exemestane group versus 9% in the placebo group (P = 0.01). Yet, clinical skeletal fractures and new-onset osteoporosis were not reported to be more frequent with exemestane compared with placebo during the course of this trial. Endocrine side effects seen in excess with exemestane versus placebo consisted of hot flashes (40% vs. 32%, respectively; P < 0.001), fatigue (23% vs. 21%, respectively; P = 0.03), and insomnia (10% vs. 8%, respectively; P = 0.04). Diarrhea and nausea were also significantly elevated with exemestane compared to placebo (5% vs. 3% and 7% vs. 5%, respectively). No significant difference was seen in cancers other than breast cancer. Although most toxicity was reported to be grade 1 or 2, a substantial number of cases of hot flashes, musculoskeletal arthritis, and joint pain were scored as grade 3 (33). This high degree of discomfort is of concern because it could potentially impair compliance with long-term preventive use of exemestane by healthy women. Participants were queried regarding health-related Physical and Mental Component Scores (SF-36) and menopause- specific quality-of-life (MENQOL) elements. No overall differences between the two groups were noted, despite exacerbation of menopausal symptoms with exemestane versus placebo.
Critique of MAP.3
Reassuring Outcomes
Several reassuring outcomes emerged from the MAP.3 trial. Among the incident invasive breast cancers, no increase was observed in ER-negative cancers with exemestane treatment relative to placebo (Table 1), and a favorable trend toward a decreased incidence of HER2-positive cancers was noted with the drug compared with placebo. In addition, the treatment arm showed only one-third the number of node-positive breast cancers compared with the placebo group. Toxicity with exemestane in MAP.3 was moderate, as expected based on data generated in the adjuvant trials using AIs. New-onset osteoporosis, fracture rate, and musculoskeletal complaints (arthralgia, myalgia, tendinitis, carpal tunnel syndrome), which were elevated in the adjuvant trials, were either not increased or increased to a lesser extent with exemestane in MAP.3. Measurements using the SF-36 as well as the MENQOL scores indicated that overall healthrelated quality of life did not differ between the exemestane and placebo groups. This finding in the face of increased adverse side effects in some of the musculoskeletal-related categories is promising.
Strengths
The MAP.3 trial is the first definitive study addressing the effect of an AI as a primary preventive intervention for breast cancer in high-risk women. The importance of the MAP.3 findings is highlighted by the fact that more than three-fourths of breast cancers are ER positive, accounting for the majority of breast cancer–related deaths (Table 2) (1). The contralateral breast cancer data from the adjuvant IES trial provided a strong rationale for MAP.3, which built on these contralateral breast data by posing the parallel question for first primary breast cancers as a primary endpoint. Furthermore, the steroidal structure of exemestane suggested that it might have androgenic properties that could potentially counteract any antiestrogenic properties in bone (10) and possibly even on sexual function and menopausal symptoms. The data on the anticipated benefits in bone are inconsistent, however, with clinical data generally not reflecting the positive results in animals (11, 12).
Table 2. MAP.3 Exemestane Prevention Trial: strengths and weaknesses.
| Strengths | Weaknesses |
|---|---|
| Three-fourths of breast cancers ER positive and account for most breast cancer deaths | Not including bone measurements (DXA scan) as predetermined endpoints, instead relying on self-report |
| Strong rationale based on contralateral breast cancer reduction in adjuvant trials | |
| Placebo-controlled design | Lack of active comparator (e.g., raloxifene) to determine best hormonal strategy: no estrogen at all vs. best balance between agonistic and antagonistic effects |
| Eligibility used a pragmatic and clinically relevant algorithm: | Loose definition of “high risk,” especially including all women ≥60 years of age, even those with Gail model risk ≤1.66% |
| 1. age ≥60 y | |
| 2. Gail model | |
| 3. history of IEN | |
| Fast recruitment | Study immaturity: powered for 38 invasive cancers for final analysis with 3-year recruitment +1.2-y follow-up. Although technically acceptable, probably not clinically meaningful. Follow-up too short for safety and risk:benefit assessment |
| Main findings: predictable high activity and excellent safety/tolerability profile | Crossover to exemestane is being offered to women on placebo. Resulting contamination diminishes the value of follow-up, making MAP.3 a large proof-of-principle trial (activity vs. efficacy). |
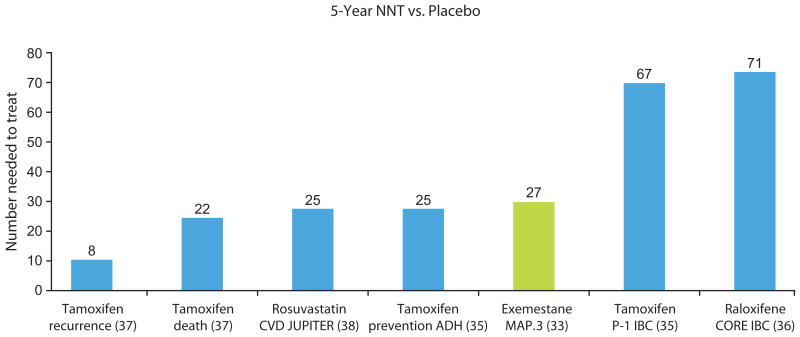
The strongest feature of the MAP.3 trial design is that it follows a prospective randomized double-blind placebocontrolled design. The eligibility criteria, which centered on age, elevated 5-year Gail model risk, and prior diagnosis of IEN, were anticipated to enrich the participant cohort for women at increased risk of breast cancer and were comparable with criteria used in prior primary prevention trials. A major advantage to the risk-reducing properties of exemestane is the relatively small NNT to prevent one breast cancer (94 at 3 years, with a projection of 26 at 5 years), although, as discussed previously, the short follow-up in MAP.3 necessitates caution in applying these projections. This compares favorably with the SERMs tested for primary prevention in the NSABP P-1 tamoxifen (35) and Continuing Outcomes Relevant to Evista (CORE) raloxifene (36) trials, in which NNT is approximately 70 (Fig. 3); compare to tamoxifen for recurrence with NNT = 8; ref. 37). In the NSABP P-1 trial, the highest-risk women, those with a history of ADH, who benefited more than any other risk class from tamoxifen, showed a 5-year NNT of 25. In the cardiovascular realm, where pharmaceutical prevention is widely accepted, NNT with the lipid-lowering drug rosuvastatin was 25 for prevention of myocardial infarction, stroke, and related disease outcomes (38). This NNT value was comparable with the values previously reported for other statins in a similar primary prevention setting (5-year NNTs ranging from 40 to 70). Thus, in terms of NNT to prevent the disease outcome of interest, breast cancer, exemestane is comparable with the most cost-effective interventions that are widely utilized in current practice. An argument commonly used to challenge the validity of primary prevention of cancer with drug interventions is that an unacceptably large number of individuals must be treated in order to prevent a single cancer. Ironically, the most commonly used preventive agent, aspirin, has an NNT in the range of 300 to 400 in order to prevent a single cardiovascular disease outcome in the primary prevention setting (38, 39).
Figure 3.
Comparison of NNT in primary prevention trials in oncology and cardiology. Abbreviations: CVD, cardiovascular; IBC, invasive breast cancer; JUPITER, Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin.
Weaknesses
Approximately 40% of participants exhibited an increased 5-year Gail model risk with only a small percentage eligible by virtue of biopsy findings, leaving the majority of women entered into MAP.3 based on age only. The median age in this study was 62.5 years, implying a median risk at baseline that is only slightly above minimum for eligibility. Implicit in the MAP.3 eligibility requirements is that women age 60 and above without a 5-year Gail risk ≥1.66% could also enter this trial and likely contributed to the lower overall risk level of trial participants (2.3% median 5-year Gail risk score) compared with prior phase III prevention trials (Table 3). The actual outcomes in MAP.3 showed an annual incidence rate of 5.5 per 1,000 women in the placebo arm. By contrast, the incidence rate was 6.8/1,000 in the NSABP P-1 (40) and 6.7/1,000 in the IBIS-I (41) trials. Whereas the inclusion of all women ≥60 is a pragmatic approach that enlarges the eligible population, allowing for rapid recruitment, it lowers the global risk level, potentially attenuating the event rate and ultimately the HR between the two arms. As justification for reliance on a placebo comparator, the MAP.3 authors interpret the 2002 ASCO Technology Assessment of drugs for breast cancer risk reduction as concluding that tamoxifen lacks global health benefits [the Study of Tamoxifen and Raloxifene (STAR) (42, 43) was not yet completed when this assessment was published] and leading to the recommendation that future trials include a placebo as the comparator arm. Yet, the actual wording in the 2002 assessment merely states that placebo controls are appropriate, leaving open as reasonable selection of an active preventive agent as a comparator for the experimental intervention (44). The actual reason that investigators, including those conducting MAP.3, have chosen to compare their experimental risk-reducing agents to inactive placebo is that a noninferiority trial, as was done with the STAR trial (42, 43), requires a very large participant cohort size, with the consequent cost in monetary and human resources.
Table 3. Comparison of baseline variables and outcomes in phase III trials of SERMs and AIs.
| Study | NSABP P-1: BCPT | IBIS-I | Royal Marsden | Italian Tamoxifen Trial | NSABP P-2: STAR | NCIC MAP.3 |
|---|---|---|---|---|---|---|
| Drug | Tamoxifena vs. placebo (ref. 35) | Tamoxifena vs. placebo (ref. 51) | Tamoxifena vs. placebo (ref. 78) | Tamoxifena vs. placebo (ref. 79) | Raloxifeneb vs. tamoxifen (ref. 43) | Exemestane vs. placebo (ref. 33) |
| Mean or median FU | 74-mo mean FU | 96-mo median FU | 13-y median FU | 11-y mean FU | 81-mo median FU | 35-mo median FU |
| Number randomized/mean or median age at randomization | 13,388/NA | 7,145/50.7 y mean | 2,494/2,471 analyzed/47 y medianh | 5,408/51 y median | 19,747/58.5 y mean (postmenopausal) | 4,560/62.5 y median (postmenopausal) |
| Risk level of participants (% participants at given risk score level) | 5-y riskd: ≤2% (∼25%) 2.01%–3% (∼31%) 3.01%–5% (∼26.6%) ≥5% (∼17.3%) | 10-y riskf: <2% (∼1.5%) 2%–3% (∼3.5%) 35% (∼24%) 5%–10% (∼55%) >10% (∼16%) | NAi | NAj | Mean 5-y riskd: = 4.03% | Median 5-y riskd: = 2.3% |
| Primary endpointc | Invasive BC incidence: RR = 0.57 (95% CI, 0.46–0.70; P < 0.001) | BC incidence (invasive + DCIS): RR = 0.73 (95% CI, 0.58–0.91; P = 0.004) | Invasive BC: HR = 0.78 (95% CI, 0.58–1.04; P = 0.1) | BC incidence: RR = 0.84 (95% CI, 0.60–1.17) | Invasive BC incidence: RR = 1.24 (95% CI, 1.05–1.47; P = 0.01) | Invasive BC: HR = 0.35 (95% CI, 0.18–.0.70; P = 0.002) |
| Secondary endpointsc | ||||||
| Noninvasive BC | Total noninvasive (DCIS + LCIS): RR = 0.63 (95% CI, 0.45–0.89; P = 0.008) | NA | NA | Noninvasive: OR = 1.51 (95% CI, 0.54–4.24; P = 0.4) | Total noninvasive (DCIS + LCIS + mixed): RR = 1.22 (95% CI, 0.95–1.59; P = 0.12) | NA |
| DCIS | NA | RR = 0.63 (95% CI, 0.32–1.20) | OR = 1.56 (95% CI, 0.67–3.61; P = 0.4) | NA | RR = 1.22 (95% CI, 0.95–1.69) | HR = 0.65 (95% CI, 0.28–1.51; P = 0.31) |
| Other groupings that include noninvasive BC | NA | NA | Any BC: HR = 0.84 (95% CI, 0.64–1.10; P = 0.2) | NA | LCIS: RR = 1.02 (95% CI, 0.61–1.70) Mixed DCIS + LCIS: RR = 2.11 (95% CI, 0.86–5.64) | ADH, ALH, LCIS: HR = 0.36 (95% CI, 0.11–1.12; P = 0.08); Invasive BC + DCIS: HR = 0.47 (95% CI, 0.27–0.79; P = 0.004) |
| BC ER status: ER-positive | RR = 0.38 (95% CI, 0.28–0.50) | RR = 0.66 (95% CI, 0.50–0.87) | HR = 0.61 (95% CI, 0.43–0.86; P = 0.005) | RR = 0.77 (95% CI, 0.51–1.16)k | RR = 0.93 (95% CI, 0.72–1.24)l | HR = 0.27 (95% CI, 0.12–0.60; P < 0.001) |
| BC ER status: ER-negative | RR = 1.31 (95% CI, 0.86–2.01) | RR = 1.00 (95% CI, 0.61–1.65) | HR = 1.4 (95% CI, 0.7–2.6; P = 0.3) | RR = 1.10 (95% CI, 0.59–2.05)k | RR = 1.15 (95% CI, 0.75–1.77)l | HR = 0.80 (95% CI, 0.21–2.98; P = 0.74) |
| Adverse events | ||||||
| Endometrial cancer | Invasive: RR = 3.28 (95% CI,1.87–6.03) ≤49 y: RR = 1.42 (CI, 0.55–3.81) ≥50 y: RR = 5.33 (CI, 2.47–13.17) In situ: RR = 0.35 (CI, 0.01–4.36) |
RR = 1.55 (95% CI, 0.68–3.65) | HR = 2.69 (95% CI, 0.96–7.55; P = 0.06) | NA | RR = 0.55 (95% CI, 0.36–0.83; P = 0.003) | NAm |
| Thromboembolic events: overall | NA | RR = 1.72 (95% CI, 1.27–2.36) | OR = 2.55 (95% CI, 0.68–9.67; P = 0.2) | RR = 1.63 (95% CI, 1.02–2.62; P = 0.04) | RR = 0.75 (95% CI, 0.60–0.93; P = 0.007) | OR = 1.58 (95% CI, 0.61–4.08; P = 0.3) |
| Thromboembolic: pulmonary emboli | RR = 2.15 (95% CI, 1.08–4.51) | [DVT/PE: RR = 1.84 (95% CI, 1.21–2.82)]g | NA | NA | RR = 0.80 (95% CI, 0.57–1.11) | NA |
| Thromboembolic: DVT | RR = 1.44 (95% CI, 0.91–2.30) | [DVT/PE: RR = 1.84 (95% CI, 1.21–2.82)] 13) | NA | NA | RR = 0.72 (95 % CI, 0.54–0.95) | NA |
| Cardiovascular events and stroke or all CVA events | Stroke: RR = 1.42 (95% CI, 0.97–2.08) TIA: RR = 0.91 (95% CI, 0.54–1.52) Ischemic heart disease: RR = 1.03 (95% CI, 0.79–1.36) |
Stroke/CVA: RR = 1.25 (95% CI, 0.55–2.93) All cardiac events: RR = 0.99 (95% CI, 0.77–1.29) |
Stroke: OR = 0.74 (95% CI, 0.28–2.00; P = 0.6) | Cerebrovascular events total: RR = 1.78 (95% CI, 0.70–4.52; CV events: RR = 1.04 (95% CI, 0.30–3.58); CV-arrhythmia: RR = 1.73 (95% CI, 1.01–2.98) |
NA | Stroke/TIA: OR = 1.19 (95% CI, 0.53–2.66; P = 0.7) CV events: OR = 0.96 (95% CI, 0.72–1.26; P = 0.78) |
| Bone: clinical skeletal fracture | Hip, spine, radius: RR = 0.68 (95% CI, 0.51–0.92)e | All fractures: RR = 1.02 (95% CI, 0.86–1.21) | OR = 0.86 (95% CI, 0.46–1.59; P = 0.6) | NA | RR = 0.92 (95% CI, 0.69–1.22)l | OR = 1.05 (95% CI, 0.83–1.33; P = 0.72) |
| Bone: new osteoporosis | NA | NA | NA | NA | NA | OR = 1.24 (95% CI, 0.76–2.02; P = 0.39) |
| Musculoskeletal: arthritis | NA | NA | NA | NA | NA | OR = 1.19 (95% CI, 0.99–1.43; P = 0.01) |
| Joint pain | NA | NA | OR = 1.18 (95% CI, 0.82–1.70; P = 0.4) | NA | NA | OR = 1.14 (95% CI, 1.00–1.30; P = 0.04) |
| Muscle pain or cramps | NA | NA | OR = 1.70 (95% CI, 0.96–3.01; P = 0.09) | NA | NA | OR = 0.75 (95% CI, 0.60–0.94; P = 0.01) |
| Hot flashes (often or extremely bothersome) or vasomotor | NA in ref. 28; TAM: 45.7% vs. placebo: 28.7%e | [Combined gynecologic and vasomotor: RR = 1.08 (95% CI, 1.06–1.10)]g | OR = 1.99 (95% CI, 1.69–2.35; P < 0.001) | RR = 1.78 (95% CI, 1.57–2.00) | NA | Vasomotor symptoms in MENQOL OR = 1.49 (95% CI, 1.31–1.69; P < 0.001)n |
| Vaginal/gynecologic symptoms (moderately bothersome or worse) | NA in ref. 28; Vaginal discharge: TAM: 29% vs. placebo: 13%e | [Combined gynecologic and vasomotor: RR = 1.08 (95% CI, 1.06–1.10)]g | Vaginal discharge: OR = 2.23 (95% CI, 1.81–2.75; P < 0.001) | Vaginal dryness: RR = 1.14 (95% CI, 0.97–1.34); vaginal discharge: RR = 3.44 (95% CI, 2.90–4.09) | NA | Vaginal dryness: OR = 1.03 (95% CI, 0.88–1.22; P = 0.68) |
Abbreviations: BC, breast cancer; BCPT, Breast Cancer Prevention Trial; CI, confidence interval; CV, cardiovascular; CVA, cerebrovascular accident; DVT, deep vein thrombosis; FU, follow-up; NA, not available; PE, pulmonary embolus; RR, relative risk or risk ratio; TAM. tamoxifen; TIA, transient ischemic attack.
Tamoxifen was approved by the FDA in October 1998.
Raloxifene was approved by the FDA September 13, 2007.
Values for OR, RR, and HR refer to comparisons of the hormonal intervention arm with the control arm.
Five-year Gail model risk score. The Gail model score calculates breast cancer risk based on known clinical and demographic factors, including age, age at first period, age at first birth, family history of breast cancer (first-degree relative: mother, sister, or daughter), number of past breast biopsies, and number of breast biopsies that showed atypical hyperplasia. The Gail model risk assessment tool is available at http://www.cancer.gov/bcrisktool/.
These rates reflect 69 months of follow-up and were obtained from the first publication of NSABP P-1:BCPT (see ref. 40).
Predicted absolute 10-year risk in placebo group based on family history and other risk factors. These numbers were obtained from the first publication of IBIS-I (see ref. 80).
Brackets indicate a repeat entry for categories listed separately in this table but combined in the original reference.
Data from Powles et al. (81).
Risk based primarily on family history, not on Gail model score.
Participants were not selected for study based on their risk of developing breast cancer. All participants had had a hysterectomy; the majority (53%) had hadboth ovaries removed during hysterectomy and were therefore at slightly less than normal risk of breast cancer.
Data from Veronesi et al. (ref. 79, Supplementary Table S1).
These values for ER status and fracture rates are from the initial analysis of NSABP P-2:STAR (see ref. 42). ER status and fracture rates are not addressed in the 2010 Update of STAR (see ref. 43).
Endometrial cancers are not addressed in this paper (33). However, the Supplementary Table S2 lists “gynecological cancers” as exemestane (5/2,240) vs. placebo (8/2,248).
Data from Goss et al. (ref. 33, Supplementary Table S3, MENQOL assessment).
A key weakness in MAP.3 was the failure to prospectively incorporate into the study design a systematic reporting of critical bone endpoints, such as new-onset osteoporosis and fractures. As a result, recording of these adverse outcomes relied on self-reporting that an event occurred and possibly self-reporting regarding the nature of the event. Furthermore, although all radiographic reports were said to have been reviewed centrally (33), it is not clear whether routine central review was carried out for reports of either dual-emission X-ray absorptiometry (DXA) scans or radiologic images of fractures. Even if such review took place, the driving force behind obtaining these reports and images relied on participant self-reporting, which may introduce bias and certainly is subject to underreporting. Although the required on-study DXA scans revealed a baseline level of osteoporosis of about 13%, newonset osteoporosis during the study was reported to be approximately 1.5%, with no difference between the arms. Similarity between the two arms was also seen in the comparable percentages of women who were reported as newly receiving bisphosphonates. The prevalence of new osteoporosis was unexpectedly low compared with data from the IES (7.3% with exemestane at a 55.7-month median follow-up) (16). This low level is likely to be the result of a lack of structured recording of this important side effect, leading to underreporting with possible ascertainment bias. Furthermore, the quality-of-life (QOL) measures (SF- 36, MENQOL) may not adequately capture the full musculoskeletal symptom-related experience of women taking exemestane in MAP.3.
The major problem with the 2011 report of the MAP.3 trial is the lack of maturity of the data. Even though the statistical design technically allowed for a minimum of 38 invasive breast cancer events, giving an estimated HR of 0.35, to determine significance for the primary endpoint, from a clinical perspective this absolute number of events is of limited relevance. Had the trial been designed to continue until a clinically more meaningful number of breast malignancies had occurred, the results would have informed clinical practice to a greater extent. In this sense, MAP.3 is an excellent large proof-of-principle prevention trial. The short 35-month duration of this trial also leaves unanswered questions about long-term toxicity, which in a prevention setting is as important as efficacy. Concerns about long-term toxicity are twofold. First, a drug or its biologic effects may accumulate over time, resulting in toxic effects not seen early in the treatment period. Second, and more important, noncritical but bothersome toxicities such as musculoskeletal events, hot flashes, and fatigue, all of which were significantly elevated with exemestane in the MAP.3 trial, are likely to lead to noncompliance. This is especially true among healthy women who are less motivated to adhere to drug therapy in the face of adverse side effects than are women with cancer. The short follow-up with unblinding also allows for the availability of the intervention drug exemestane to women on the placebo arm, such that further posttrial follow-up will suffer from contamination, diminishing the difference between the arms.
Where do the MAP.3 Data Fit into Current Knowledge of Agents for Breast Cancer Prevention?
Exemestane and AIs Compared to SERMs for Primary Prevention of Breast Cancer
A 5-year intervention with SERMs (tamoxifen, raloxifene) is associated with an approximately 40% reduction of invasive breast cancer overall at 10 years (45, 46). When combined, the data from the four phase III tamoxifen-versus- placebo prevention trials show that the risk of breast cancer is reduced by nearly 40% (45), that is, 38% (95% CI, 28–46; P < 0.0001) when analyzed by a fixed-effect model (45), with a 48% (95% CI, 36–58; P ≤ 0.0001) reduction in ER-positive and no effect on ER-negative breast cancers (45) (Table 3). When trials of raloxifene in women with osteoporosis, including the Multiple Outcomes of Raloxifene Evaluation (MORE), CORE, and Raloxifene Use for the Heart (RUTH) trials, were subjected to a meta-analysis, a 59% reduction in ER-positive breast cancer (RR =0.41; 95% CI, 0.27–0.62) was demonstrated (46). However, direct comparison between tamoxifen and raloxifene in the STAR trial showed a greater efficacy of tamoxifen (42, 43). A substantial risk reduction has also been observed with lasofoxifene (HR = 0.21; 95% CI, 0.08–0.55; P = 0.001) (2, 3) and arzoxifene (HR = 0.44; 95% CI, 0.26–0.76; P < 0.001) (4). Compared with the U.S. Food and Drug Administration (FDA)–approved SERMs, the MAP.3 data suggest considerably greater efficacy with the AI exemestane for cancer prevention, with a 65% reduction in risk of invasive breast cancer and a 73% reduction in risk of ERpositive breast cancer in high-risk women (33). This striking advantage of exemestane over the two FDA-approved SERMs concurs with the results of a combined analysis of adjuvant trials of all three AIs that shows an overall reduction in ER-positive contralateral breast cancers of 40% to 0% with AIs over and above tamoxifen (47). In view of the benefit already conferred by tamoxifen, these data suggest a net benefit with the AIs of 70% to 80% reduction in new primary ER-positive cancers in the contralateral breast relative to no treatment.
The additional advantage that AIs in general have over SERMs is their relative lack of adverse side effects. Despite the adverse effect of estrogen depletion on BMD, fracture rate, and musculoskeletal symptoms, the global balance between benefits and toxicities generally supports the use of AIs over tamoxifen for breast cancer treatment and prevention. This is seen in the QOL analyses in the MAP.3 trial. Also in the ATAC trial at 68 months median follow-up, the Global Index of the Women's Health Initiative (HR = 0.85; 95% CI, 0.77–0.94; P = 0.001) and the Global Index of Disease-Free Survival and Serious Adverse Events (HR = 0.88; 95% CI, 0.82–0.94; P = 0.0004) both favor anastrozole over tamoxifen as a result of significantly fewer events in the anastrozole arm (48). This evidence for a superior benefit:toxicity balance for AIs over tamoxifen must be taken with caution, however, given the subjectivity inherent in weighing the importance of different toxicities against each other.
Implications for Research Trials of Other AIs Needed to Confirm MAP.3 Results
Although the MAP.3 data are promising, the institution of AIs for primary breast cancer prevention requires confirmatory results from additional phase III trials. A second AI, anastrozole, is currently being tested in the International Breast Cancer Intervention Study-II (IBIS-II) for its ability to reduce the risk of invasive breast cancer in postmenopausal women at increased risk of disease. The overall IBIS-II design comprises two independent “arms,” one testing anastrozole in comparison with tamoxifen in women with DCIS and the other, a close parallel to the MAP.3 study, testing this AI versus placebo in postmenopausal women at increased risk of breast cancer. The eligibility criteria for this “high-risk” portion of IBIS-II are similar to those used in IBIS-I, including risk based on family history, history of benign breast biopsies, LCIS and/or atypical hyperplasia, and nulliparity. All women must be age 35 to 70 years and postmenopausal. Exclusion criteria include a DXA T score < −4 or more than two fragility fractures. An important addition is eligibility based on high breast density (increased density in >50% of the breast), a recently established risk factor for developing invasive disease. Concern about anticipated bone toxicities led to inclusion of a prospectively designed supplementary bone study in women on both the placebo and the anastrozole arms. Based on baseline DXA scan measurements, 1,000 women are being entered into three groups: (1) those who do not receive the bisphosphonate risedronate (BMD > −1.5); (2) those who will be randomized to placebo versus risedronate (BMD of −1.5 to −2.5); or (3) those who will be administered risedronate (BMD < −2.5). Additional supplementary studies will examine issues of cognitive function and QOL, DCIS pathology, genetic studies in blood lymphocytes, serum levels of hormones and lipid profiles, and endometrial biopsies. As of October 13, 2011, 6,564 eligible women had been randomized, and the plan is to complete accrual at the end of 2011. The results of the IBIS-II trial are eagerly anticipated, because they will provide a comparator to the exemestane data generated by MAP.3. In both trials, however, the lack of a comparator arm utilizing a proven risk-reducing agent deprives researchers of the ability to evaluate the new, promising breast cancer prevention agents against the FDA-approved SERMs.
Clinical Use of Biomarkers in Cancer Prevention Trials: Early Selection of Responders versus Nonresponders
Biomarkers of risk and as surrogates for clinical outcomes have routinely been incorporated into the design of the smaller phase II prevention trials (49, 50). Initial forays are being made into the incorporation of surrogate endpoint biomarkers (SEB) into phase III trials, as seen in the IBIS-I tamoxifen-versus-placebo trial (51), in which the biomarker endpoint of mammographic density was retrospectively analyzed in a nested case-control study (52). When compared with all the women in the placebo group, the 46% of women treated with tamoxifen who experienced a ≥10% reduction in breast density over 12 to 18 months exhibited a 63% reduction in risk of breast cancer (OR = 0.37; 95% CI, 0.20–0.69; P = 0.002). By contrast, those who were treated with tamoxifen but experienced less than a 10% reduction in breast density exhibited no risk reduction (OR = 1.13; 95% CI, 0.72–1.77, P = 0.60). Though relatively short, the time frame of the mammographic density response allowed prediction of the actual clinical endpoint of interest, invasive breast cancer. In much the same manner, SEBs, as validated biomarkers of response, could be incorporated prospectively into phase III trials to document early response to the drug or other intervention. In this manner, the biomarker could be used to indicate who at an individual level actually responds to an already approved agent. The goal is to screen eligible women for short-term biomarker response to confirmed agents, thereby avoiding prolonged administration of a preventive drug, with possible adverse sequelae, to individuals predicted not to benefit from it.
Dose, Schedule, and Duration of Therapy
The optimal biologic dose remains a poorly investigated component of preventive therapy, generally having been imported from treatment trials or trials addressing noncancer endpoints. Lower, presumably less toxic, but equally efficacious doses have been implemented in phase II trials. DeCensi and colleagues have shown that low-dose tamoxifen (1 mg/d, 5 mg/d, or 20 mg/wk) compares favorably with the standard 20 mg/d dose in terms of downward modulation of Ki-67 in breast cancer tissue (53), several blood biomarkers [including insulin-like growth factor-I (IGF-I), low-density lipoprotein (LDL), and ultrasensitive C-reactive protein (53, 54)] and mammographic density (54, 55). In an observational study addressing clinical endpoints, low-dose tamoxifen (either 5 mg/d or 20 mg/wk) was associated with improved disease-free survival in women treated surgically for ductal IEN that was ER positive (56). Early dose-finding studies examined changes in levels of estrogen and other hormones in response to exemestane doses including 0.5, 1.0, 2.4, 5, 12.5, and 25 mg/d (57, 58). Although some estrogen suppression has been seen at all doses, maximum inhibition of estrogen production occurs at 25 mg/d or 10 mg/d depending on the study (59, 60), while the lowest doses tested, 0.5 and 1.0 mg, do not adequately suppress circulating estrogens (58). Based on these historical observations, future prevention trials of exemestane should explore more thoroughly the pharmacologic and pharmacodynamic effects of doses lower than the standard 25 mg/d used in MAP.3 and in the treatment setting. Similarly, schedule and duration of therapy remain unexplored. These temporal components are particularly important in the prevention setting where long-term uninterrupted use may increase toxicity in healthy individuals.
Interaction of Obesity with AI Effects
Dose modulation of AIs assumes importance when results of AI trials are analyzed in terms of obesity. Obesity is a known risk factor for breast cancer, particularly ERpositive cancer (61), as well as recurrence and decreased survival in postmenopausal women with breast cancer (62–64). These associations are thought to be due to increased adipose tissue coupled with excess aromatase in the adipose tissue of obese women (65, 66), accounting for the higher circulating estrogen concentrations observed in these women (67). The elevation of aromatase, in turn, appears to be related to increased levels of proinflammatory mediators, including TNF-α, interleukin-1β, COX-2 activity, and the accumulation of crownlike structures consisting of necrotic adipocytes surrounded by macrophages in the adipose tissue, including breast adipose tissue, of obese women (68).
In the ATAC trial, a significantly higher rate of breast cancer recurrence was observed in women with a high body mass index [(BMI) >35 kg/m2) compared with those with a low BMI (<23 kg/m2), with an HR = 1.39 (95% CI, 1.06–1.82; Pheterogeneity = 0.03) (69). Furthermore, although overall anastrozole was associated with a 27% lower rate of recurrence than tamoxifen (HR = 0.73; 95% CI, 0.63–0.83; P < 0.001) and decreased recurrence with the AI was seen at all BMI levels, aromatase inhibition at the administered dose (1 mg/d) was less effective in preventing breast cancer recurrences in obese women (HR = 0.84; 95% CI, 0.61–1.14) than in thinner women (HR = 0.64; 95% CI, 0.45–0.91). The significant elevation of musculoskeletal and menopausal symptoms in ATAC participants taking anastrozole versus tamoxifen (OR = 1.25; 95% CI, 1.11–1.40) was more pronounced in women with a BMI >30 kg/m2 compared to those with BMI <25 kg/m2 (OR = 1.32; 95% CI, 1.14–1.53; P, 0.0001) (48, 70). Although obesity is associated with joint symptoms independent of endocrine treatment (71), the ATAC findings suggest that a greater decrease in estrogen levels with aromatase inhibition in these women may also have contributed to their increased reporting of joint symptoms. The application of AIs to cancer prevention requires that future prevention trials incorporate into their design an investigation of the impact of adjusting AI dose to BMI so as to optimize efficacy and toxicity outcomes.
Communicating Prevention Trial Results to High-Risk Women and Their Physicians
The MAP.3 data offer another example of evidencebased testing of a putative preventive agent for cancer. For breast cancer, comparable evidence-based data have been generated for tamoxifen (40) and raloxifene (42) in high-risk women. Despite such strong evidence and FDA approval in the United States of both agents for risk reduction of breast cancer, and their endorsement for this purpose in specific categories of women by the American Society of Clinical Oncology Clinical Practice Guidelines committee (72), the acceptability of these drugs to the community of high-risk women and their primary care physicians has been low (73–75). The limited uptake of drug interventions for prevention in oncology has been attributed primarily to concern about drug toxicities, particularly with tamoxifen, and a perceived unfavorable balance between risks and benefits. A restrictive list of attributes (being premenopausal, limiting the options to tamoxifen; very high risk due to a history of ADH, LCIS, or DCIS; not at risk of and no history of thromboembolic disease; and hysterectomy if postmenopausal) are regarded as contributing to a favorable risk:benefit balance, making a woman a good candidate for preventive drug therapy (76), but these complex issues may be challenging to address in a routine office visit.
This reluctance is compounded by the absence of experience with oncology drugs among the internists, gynecologists, and family practitioners who generally manage high-risk women, but who may be hesitant to prescribe tamoxifen, which they perceive as a “cancer drug” with challenging side effects. These explanations for the low uptake in community practice of proven preventive approaches have also been noted in studies of psychologic and clinical factors contributing to the willingness of women to participate in clinical trials of preventive tamoxifen (77).
Implications for Preventive Care
The results of the MAP.3 trial strongly support integrating the AI exemestane into the armamentarium of breast cancer risk-reducing agents. Currently, tamoxifen is the standard for premenopausal women, given the lesser tamoxifen toxicity in this group. Based on the relative safety profiles observed for tamoxifen and raloxifene in the STAR trial data (42, 43), raloxifene might be considered the standard in postmenopausal women. However, the update of STAR showed raloxifene to retain only 76% of the effectiveness of tamoxifen for preventing invasive breast cancer (43). Exemestane not only joins raloxifene as an acceptable preventive intervention in these postmenopausal highrisk women but also, based on the MAP.3 data, shows clear advantages in terms of efficacy and possibly toxicity for use in this population. The crucial issue at this juncture is to develop strategies for communicating with community physicians and high-risk women about the value of risk reduction for breast cancer and promoting a better understanding of the risk:benefit balance for each of the available preventive agents.
Acknowledgments
Grant Support: A. DeCensi's work was supported in part by a contract from the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Footnotes
Note: This article was presented orally at the annual meeting of the American Society of Clinical Oncology, Chicago, June 5, 2011.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103:1397–402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–96. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 3.LaCroix AZ, Powles T, Osborne CK, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010;102:1706–15. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, McClung M, Reginster JY, et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J Bone Miner Res. 2011;26:397–404. doi: 10.1002/jbmr.191. [DOI] [PubMed] [Google Scholar]
- 5.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell KL. Are all aromatase inhibitors alike? Breast Cancer Res Treat. 2008;112(1):S35–43. doi: 10.1007/s10549-008-0233-9. [DOI] [PubMed] [Google Scholar]
- 7.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29:2342–9. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giudici D, Ornati G, Briatico G, Buzzetti F, Lombardi P, di Salle E. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): a new irreversible aromatase inhibitor. J Steroid Biochem. 1988;30:391–4. doi: 10.1016/0022-4731(88)90129-x. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Qi S, Josse RG, et al. The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats. Bone. 2004;34:384–92. doi: 10.1016/j.bone.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Goss PE, Qi S, Cheung AM, Hu H, Mendes M, Pritzker KP. Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized rats. Clin Cancer Res. 2004;10:5717–23. doi: 10.1158/1078-0432.CCR-04-0438. [DOI] [PubMed] [Google Scholar]
- 11.Lonning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23:5126–37. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 12.Cigler T, Richardson H, Yaffe MJ, et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat. 2011;126:453–61. doi: 10.1007/s10549-010-1322-0. [DOI] [PubMed] [Google Scholar]
- 13.Arun B, Dunn BK, Ford LG, Ryan A. Breast cancer prevention trials: large and small trials. Semin Oncol. 2010;37:367–83. doi: 10.1053/j.seminoncol.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 15.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 16.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–70. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 18.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–62. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007;25:2664–70. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 20.Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845–53. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]
- 21.Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23:5138–47. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 22.van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377:321–31. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 23.Boccardo F, Rubagotti A, Guglielmini P, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006;17(7):S10–14. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 24.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 25.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 26.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 27.Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomized controlled study. Lancet Oncol. 2007;8:119–27. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 28.Richardson H, Johnston D, Pater J, Goss P. The National Cancer Institute of Canada Clinical Trials Group MAP.3 trial: an international breast cancer prevention trial. Curr Oncol. 2007;14:89–96. doi: 10.3747/co.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase- 1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–60. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 30.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001;84:1188–92. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 32.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 33.Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breastcancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 34.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. Br Med J. 1999;319:1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 36.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–61. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 37.Moore A, McQuay H. Tamoxifen trials, tribulations and truths. Bandolier: evidence-based thinking about health care. 1998;5:6–7. Available from : http://www.jr2.ox.ac.uk/Bandolier. [Google Scholar]
- 38.Ridker PM, MacFadyen JG, Fonseca FA, et al. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) Circ Cardiovasc Qual Outcomes. 2009;2:616–23. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 39.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascularn events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–13. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 40.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 41.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomized prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 42.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 43.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chlebowski RT, Col N, Winer EP, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol. 2002;20:3328–43. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 46.Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–98. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636–43. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for earlystage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–43. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 49.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer—a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 50.Kelloff GJ, Crowell JA, Hawk ET, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem Suppl. 1996;26:54–71. doi: 10.1002/jcb.240630705. [DOI] [PubMed] [Google Scholar]
- 51.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 52.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 53.Decensi A, Robertson C, Viale G, et al. A randomized trial of lowdose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–90. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 54.Decensi A, Gandini S, Serrano D, et al. Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol. 2007;25:4201–9. doi: 10.1200/JCO.2006.09.4318. [DOI] [PubMed] [Google Scholar]
- 55.Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749–56. doi: 10.1200/JCO.2008.19.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerrieri-Gonzaga A, Botteri E, Lazzeroni M, et al. Low-dose tamoxifen in the treatment of breast ductal intraepithelial neoplasia: results of a large observational study. Ann Oncol. 2010;21:949–54. doi: 10.1093/annonc/mdp408. [DOI] [PubMed] [Google Scholar]
- 57.Zilembo N, Noberasco C, Bajetta E, et al. Endocrinological and clinical evaluation of exemestane, a new steroidal aromatase inhibitor. Br J Cancer. 1995;72:1007–12. doi: 10.1038/bjc.1995.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajetta E, Zilembo N, Noberasco C, et al. The minimal effective exemestane dose for endocrine activity in advanced breast cancer. Eur J Cancer. 1997;33:587–91. doi: 10.1016/s0959-8049(96)00494-7. [DOI] [PubMed] [Google Scholar]
- 59.Johannessen DC, Engan T, Di Salle E, et al. Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: a phase I study. Clin Cancer Res. 1997;3:1101–8. [PubMed] [Google Scholar]
- 60.Lonning PE. Exemestane: a review of its clinical efficacy and safety. Breast. 2001;10:198–208. doi: 10.1054/brst.2001.0293. [DOI] [PubMed] [Google Scholar]
- 61.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 62.Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–91. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 63.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States) Cancer Causes Control. 2002;13:741–51. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 64.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Br Med J. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–42. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 67.Zumoff B. Relationship of obesity to blood estrogens. Cancer Res. 1982;42(8 Suppl):3289s–94s. [PubMed] [Google Scholar]
- 68.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–5. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 70.Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–72. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 71.Cooper C, Inskip H, Croft P, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol. 1998;147:516–22. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 72.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19:443–6. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila) 2010;3:686–8. doi: 10.1158/1940-6207.CAPR-10-0100. [DOI] [PubMed] [Google Scholar]
- 75.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–5. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–46. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 77.Rondanina G, Puntoni M, Severi G, et al. Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol. 2008;26:1537–43. doi: 10.1200/JCO.2007.13.6739. [DOI] [PubMed] [Google Scholar]
- 78.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 79.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–37. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 80.IBIS Investigators. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 81.Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]