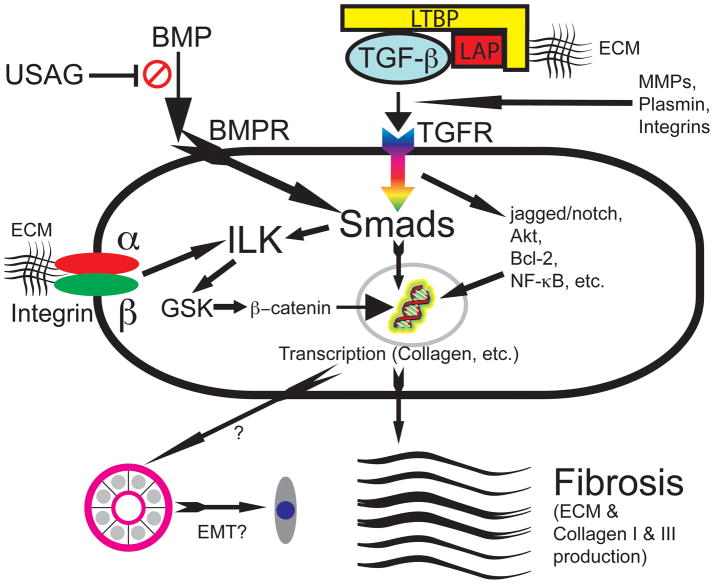
Figure 2. Important molecular mediators of fibrosis.
Transforming growth factor (TGF-β) is released through interactions with the extracellular matrix (ECM) and matrix metalloproteinases (MMPs), plasmin, and integrin; and when released from inhibition by latent TGF-β binding protein (LTBP) and latency-associated peptide (LAP), TGF-β binds the transforming growth factor receptor (TGFR), activating intracellular signals such as the Smad, jagged/notch, Akt, Bcl-2, and NF-κB pathways. These lead to nuclear transcription, ultimately culminating in collagen and ECM production and possibly leading to epithelial to mesenchymal transition (EMT). Smads also act on the integrin-linked kinase (ILK), which acts through glycogen synthase kinase (GSK) to produce β-catenin, which traverses into the nucleus to also induce transcription. The integrins (typically with α and β components [e.g., α5β6 integrin]) also act through ILK in a similar manner. Bone morphogenic protein (BMP), when binding to the BMP receptor (BMPR) also works through Smad, a process inhibited by sclerostin domain-containing protein 1 (also known as uterine sensitization-associated gene 1 [USAG-1]).[Figure adapted from [1, 229, 230].]