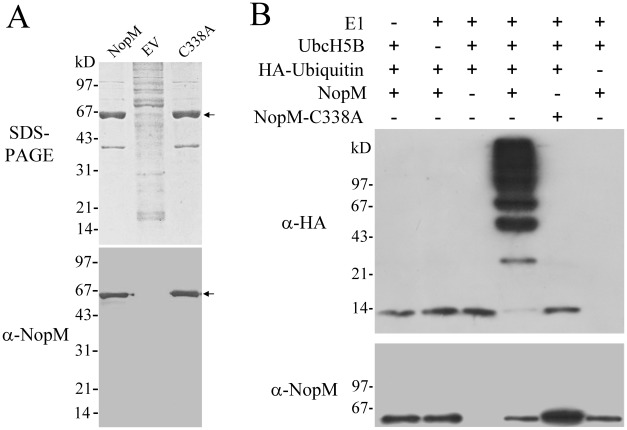
Figure 1. NopM possesses E3 ubiquitin ligase activity.
(A) Purification and immunoblot analysis of His-tagged NopM and point-mutated His-tagged NopM-C338A (marked by an arrow). Proteins purified by nickel–nitrilotriacetic acid affinity chromatography were obtained from E. coli BL21 (DE3) harboring pET-nopM (lane NopM) and pET-nopM(C338A) (lane C338A), respectively. BL21 (DE3) with the empty vector pET28b was used as a control (lane EV). The SDS-PAGE gel (0.5 µg loaded proteins) was stained with Coomassie Brilliant Blue R-250 and the corresponding immunoblot with the rabbit serum raised against NopM was developed with 3, 3′-diamino-benzidine. (B) In vitro ubiquitination reactions with indicated purified proteins followed by immunoblot analysis with chemiluminescence reagents using anti-HA and anti-NopM antibodies. Ubiquitination reactions were performed in the presence or absence of HA-tagged ubiquitin, E1, UbcH5B, His-tagged NopM and His-tagged NopM-C338A for 1 h at 37°C.