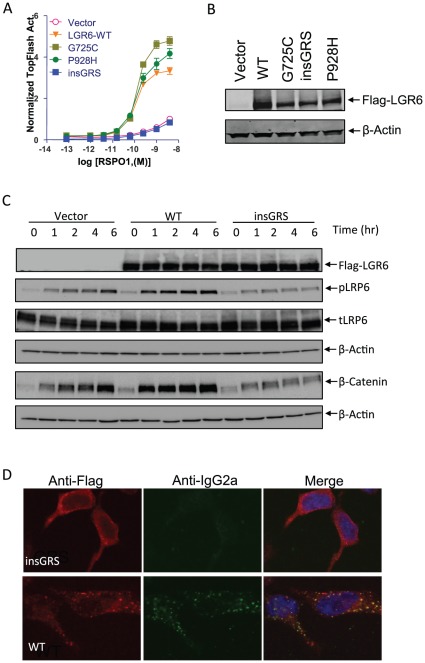
Figure 4. Functional and binding analyses of LGR6 mutants.
A, TOPFlash assay of LGR6 mutants on RSPO1-induced potentiation of Wnt/β-catenin signaling. HEK293T cells were transiently transfected with expression plasmids as indicated with a combination of super 8× TOPFlash and pRL-SV40 reporter gene constructs, and then stimulated with serial dilutions of purified recombinant RSPO1 in the presence of Wnt3a CM. All error bars are SEM (n = 4). B, Expression levels of LGR6 mutants and WT in HEK 293T cells in transient transfection paradigms. Total cell lysates were treated with Laemmli buffer for 1 hr at 37°C, fractionated by SDS-PAGE using 4–20% gels, electrophoretically transferred to nitrocellulose membrane, and then probed with anti-Flag antibody. The signal was detected by ECL Western blotting detection reagents (Amersham Biosciences). β-actin was also probed as loading control. C, Time course of changes in Wnt3a-RSPO1-induced β-catenin accumulation and LRP6 phosphorylation in vector, LGR6-WT and insGRS-overexpressing cells. HEK293 cells stably expressing vector, LGR6-WT or insGRS were stimulated with 1 ng/ml RSPO1 plus Wnt3a CM for 0–6 hrs. Total cell lysates were probed with antibodies against Flag-LGR6, phosphor-Ser1490, total LRP6, and β-actin. For the analysis of nonmembrane-bound β-catenin, the cell lysates were cleared with ConA-sepharose beads and then probed with an antibody against β-catenin as described before [9]. D, mRSPO1-Fc binding to LGR6-insGRS and WT. HEK293 cells stably expressing Flag-LGR6-WT or insGRS were incubated with mRSPO1-Fc at 37°C for 1 hr. The cells were fixed, permeabilized, and then co-stained with anti-Flag (red) and anti-IgG2a (green) antibodies to detect LGR6 and mRSPO1-Fc, respectively. Nuclei (blue) were counterstained with To-Pro-3.