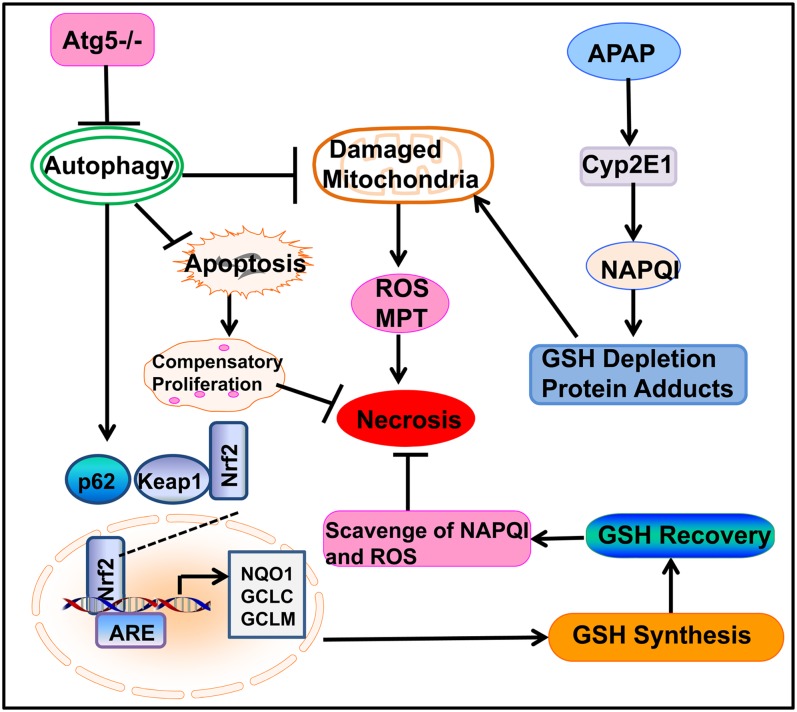
FIG. 6.
A proposed model for the loss of Atg5 in the mouse liver and the cross talk among autophagy, apoptosis, and necrosis in APAP-induced liver injury. In APAP-treated hepatocytes, APAP is first metabolized through the cytochrome P450 enzymes (mainly via CYP2E1) and generates reactive metabolites that bind to cellular and mitochondrial proteins to initiate mitochondrial damage. Damaged mitochondria can lead to necrotic cell death by inducing onset of mitochondria permeability transition (MPT) and reactive oxygen species (ROS) production. Genetic deletion of Atg5 in the mouse liver leads to the suppression of autophagy and accumulation of p62. Increased p62 proteins results in persistent activation of Nrf2 in the mouse liver by releasing the inhibition of Keap1 on Nrf2. Persistent activation of Nrf2 increases the expression of GSH synthesis enzymes, resulting in the increased recovery of GSH content in the mouse liver that further scavenges NAPQI and ROS to protect against APAP-induced liver injury. Loss of autophagy may increase the baseline hepatocytes apoptosis in the mouse liver resulting in compensatory proliferation, which may also attenuate APAP-induced liver injury by promoting liver repair and regeneration.