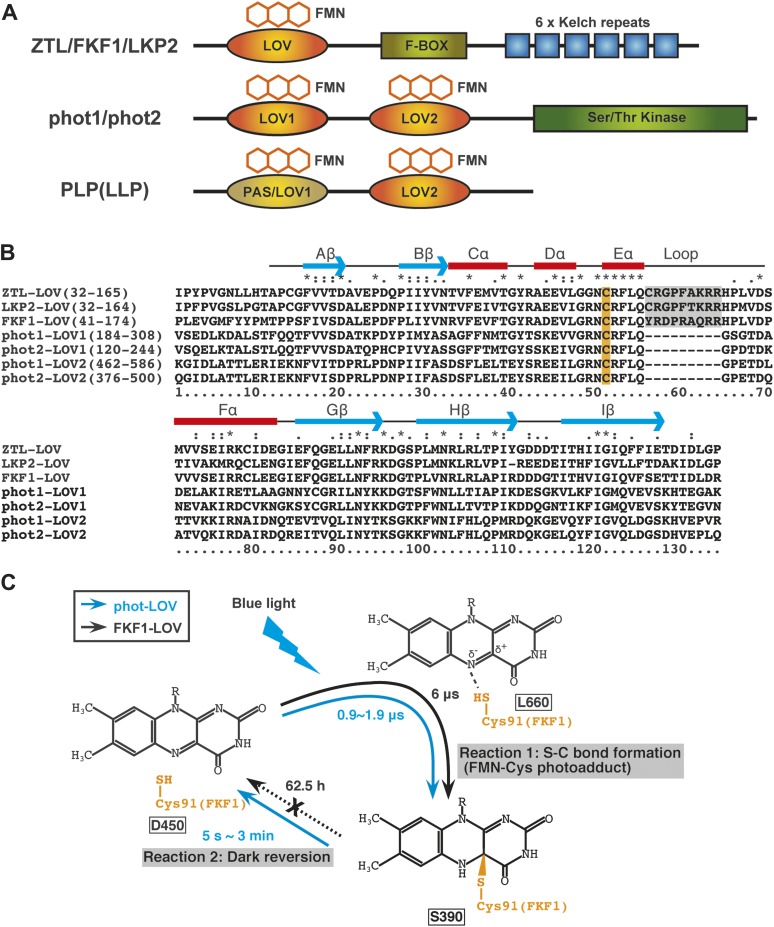
Figure 1.
The Domain Structures of ZTL/FKF1/LKP2 and Related Proteins, Amino Acid Sequence Alignments of LOV Domains, and Photochemical Properties of Phototropin and FKF1 LOV Domains.
(A) Schematic illustration of functional domains of ZTL/FKF1/LKP2 family proteins, phototropin proteins, and PAS/LOV proteins. LOV domain bound to an FMN molecule functions as a blue-light-sensing domain. The ZTL/FKF1/LKP2 family proteins possess one LOV domain at the N-terminus region followed by an F-box domain and six Kelch repeats in the C-terminal region. The phototropins contain two FMN-binding LOV domains in their N-terminal region (LOV1 and LOV2) and a serine/threonine kinase domain at the C-terminus. The PAS/LOV proteins (PLP or also called LLP) contain two LOV domains; however, the cysteine residue essential for the cysteinyl adduct formation within the first LOV domain is not always conserved in some plant species, including Arabidopsis (Kasahara et al., 2010), indicating that there is no light-induced photocycle of the LOV domain. The LOV2 domains of PLP proteins usually contain the conserved cysteine and show the blue-light-induced photocycle in vitro.
(B) Sequence alignment of several LOV domains. The alignment includes Arabidopsis thaliana ZTL, LKP2, FKF1, phot1-LOV1, phot1-LOV2, phot2-LOV1, and phot2-LOV2. Identical and similar amino acids are marked on top of the alignment by asterisks (*) and (: or .), respectively. The conserved cysteines for FMN binding are highlighted in orange. The characteristic loop regions of ZTL/FKF1/LKP2 LOV are gray-shaded. The predicted secondary structure elements are shown on top of the alignment. Arrows and boxes indicate β-strands and α-helices, respectively.
(C) Schematic representation of LOV-domain photochemistry. In darkness, the FMN chromophore is non-covalently bound in the LOV domain (L450). Light triggers the production of a reactive triplet-state flavin (L660) that leads to formation of a covalent bond between the FMN and a conserved cysteine residue in the LOV domain (S390). The photoreaction process of the phot-LOV protein (right blue arrow circuit) is fully reversible in the dark. In contrast, the FKF1–LOV protein (a black dotted arrow) shows a slow dark reversion rate. The dark reversion rate (half-lives; t1/2) of each protein is shown.