Figure 3. Differentiated GBM cells express NOTCH ligands and have reduced ability to form intracranial xenograft in mice.
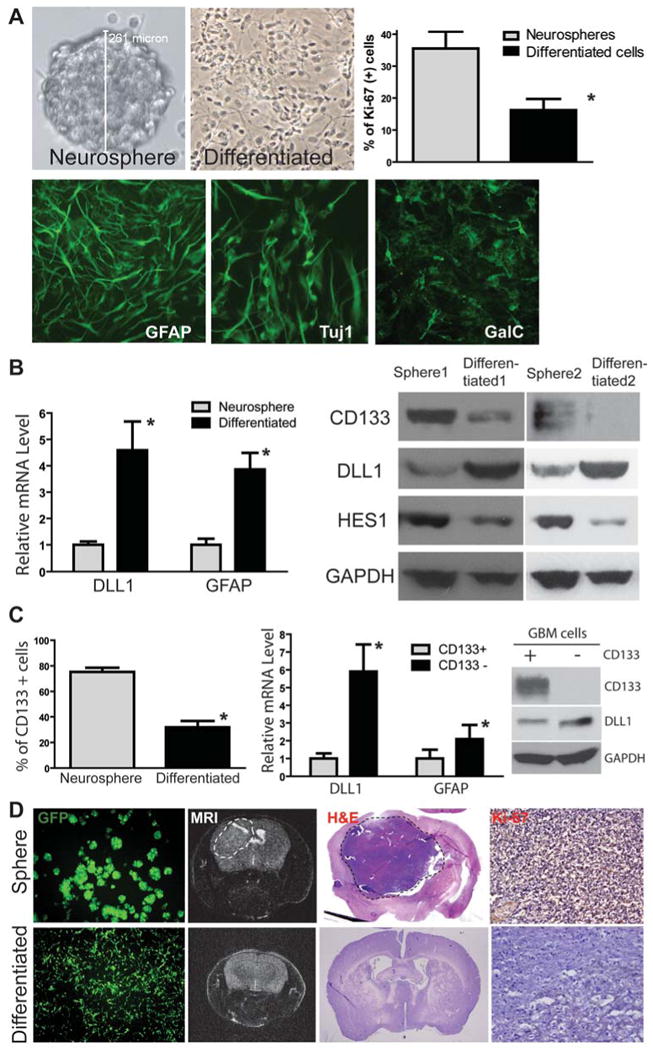
(A) HSR-GBM1 neurosphere line was forced to differentiate and grow as a mono-layer, which had reduced proliferation (*: p<0.05, t-test) and induced expression of GFAP (glial), Tuj1 (neuronal) and GalC (oligodendrocyte) markers. (B) Differentiated HSR-GBM1 cells expressed higher levels of DLL1 and GFAP at the mRNA level detected by quantitative RT-PCR (left panel). In addition, differentiated cells expressed a higher level of DLL1 and lower levels of NOTCH target HES1 and stem cell marker CD133 at the protein level as detected by Western blot in two different GBM neurosphere line HSR-GBM1 and HSR-GBM2. (C) CD133-positive population was significantly reduced in differentiated tumor cells compared with GBM neurospheres (HSR-GBM1) detected by flow cytometry (left panel). In addition, CD133-negative population in HSR-GBM1 neurospheres expressed higher levels of DLL1 and GFAP at mRNA level compared with CD133-positive population (middle panel). Up-regulation of DLL1 in CD133-negative population was also confirmed at the protein level by Western blot (right panel). (D) When GBM neurospheres or differentiated cells from HSR-GBM1 labeled with GFP using lentivirus were injected into the mouse brain, neurosphere cells formed large xenografts as detected by MRI (dash circle), whereas differentiated cells had reduced ability to form xenografts. Pathology was confirmed by H&E staining and immunostaining of the proliferation marker Ki-67.
