Figure 3. SDS-PAGE gel showed sub-saturating binding of calponin to actin.
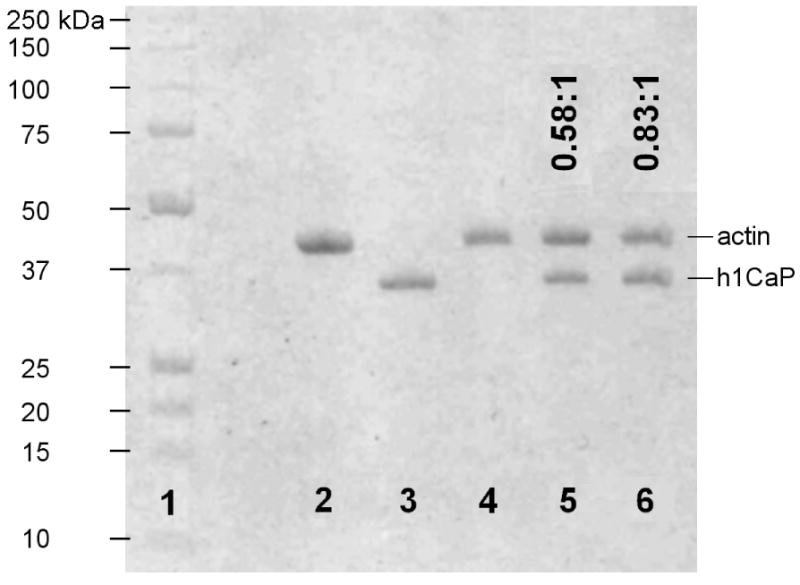
F-actin was incubated with basic calponin under conditions similar to those of the flexural rigidity and actin shearing experiments (see Materials and Methods) and ultracentrifuged at 100,000 g, and the pellet was run on a 12.5% SDS-PAGE gel. 1: BioRad standard 161-0363, with molecular weight markers shown to the left; 2: control lane with 4 μg actin; 3: control lane with 4 μg h1CaP; 4-6: 500 nM F-actin incubated and pelleted with 0, 2.5 μM, or 5 μM h1CaP. Molar binding ratios derived from densitometry measurements are indicated as h1CaP:actin above each lane. The results show that persistence length changes occurred at sub-saturating calponin binding, while filament rupture persisted to near full decoration.
