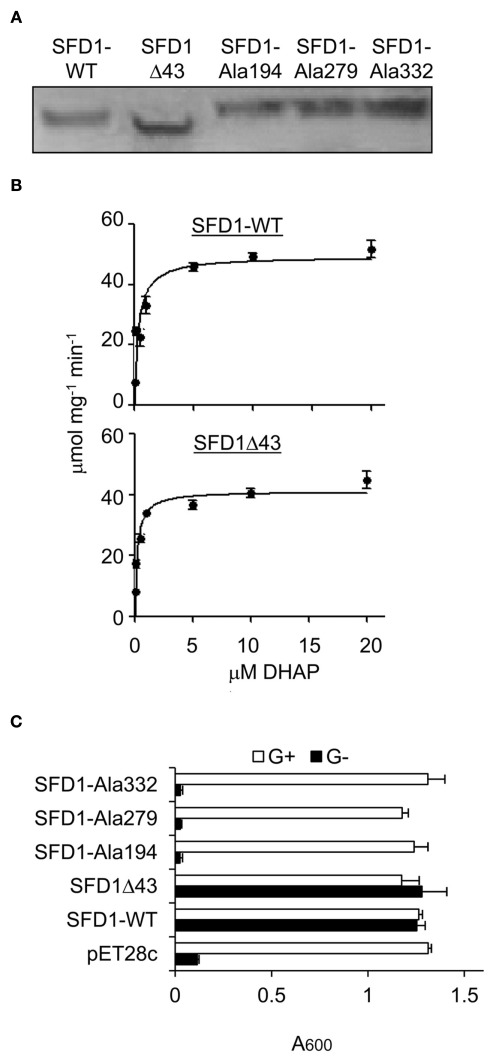
Figure 3.
DHAP reductase activity of WT and mutant SFD1 protein. (A) Immunodetection of the recombinant SFD1 proteins with anti-His-tag antibody. (B) DHAP reductase activity of the purified recombinant SFD1-WT and SDF1Δ43 proteins. DHAP reductase activity was measured as the rate of decrease in A340 nm due to oxidation of NADH to NAD+. No activity was detected with the recombinant SFD1–Ala194, SFD1–Ala279, and SFD1–Ala332 proteins. (C) Functional complementation of an E. coli G3P auxotrophic mutant by WT and mutant SFD1 proteins. Growth of the E. coli DHAP reductase-deficient strain BB20-14 expressing the WT and mutant SFD1 constructs on minimal liquid M9 medium with or without 0.1% glycerol. The empty pET28c vector-transformed E. coli provided the negative control. Cell growth was determined by measuring the optical density at A600. G+, supplemented with glycerol; G−, without glycerol.