Abstract
Background
Retinol-binding protein 4 (RBP4) is an adipokine identified as a marker of insulin resistance in mice and humans. Protein tyrosine phosphatase 1B (PTP1B) expression levels as well as other genes involved in the endoplasmic reticulum (ER) stress response are increased in adipose tissue of obese, high-fat-diet-fed mice. In this study we investigated if serum and/or adipose tissue RBP4 protein levels and expression levels of PTP1B and other ER stress-response genes are altered in obese and obese/diabetic men resident in northeast Scotland.
Methods
We studied three groups of male volunteers: (1) normal/overweight (body mass index [BMI] < 30), (2) obese (BMI > 30), and (3) obese/diabetic (BMI > 30) controlling their diabetes either by diet or the antidiabetic drug metformin. We analyzed their serum and adipose tissue RBP4 protein levels as well as adipose tissue mRNA expression of PTP1B, binding immunoglobulin protein (BIP), activated transcription factor 4 (ATF4), and glucose-regulated protein 94 (GRP94) alongside other markers of adiposity (percentage body fat, leptin, cholesterol, triglycerides) and insulin resistance (oral glucose tolerance tests, insulin, homeostatic model assessment–insulin resistance, C-reactive protein, and adiponectin).
Results
We found that obese Scottish subjects had significantly higher serum RBP4 protein levels in comparison to the normal/overweight subjects (P < 0.01). Serum RBP4 levels were normalized in obese/diabetic subjects treated with diet or metformin (P < 0.05). Adipose tissue RBP4 protein levels were comparable between all three groups of subjects as were serum and adipose transthyretin levels. Adipose tissue PTP1B mRNA levels were increased in obese subjects in comparison to normal/overweight subjects (P < 0.05); however diet and/or metformin treatment did not reverse this effect. Adipose tissue BIP, ATF4, and GRP94 expression levels were unchanged in obese and obese/diabetic subjects.
Conclusions
Human obesity results in an increase in serum but not adipose tissue RBP4 protein levels, and these are normalized in obese/diabetic subjects, which exhibit improvements in insulin sensitivity through diet or metformin treatment. However, while adipose tissue PTP1B mRNA levels increase in obese Scottish subjects, these remain high in obese/diabetics on diet or metformin treatment.
Keywords: obesity, diabetes, metformin, PTP1B, RBP4, TTR, ER stress, UPR, GRP94, BIP, ATF4, insulin resistance, glucose homeostasis
Introduction
Obesity is a major risk factor for a number of metabolic diseases, such as type 2 diabetes, cardiovascular disease, cancer, Alzheimer’s disease, and others. Recent studies clearly demonstrate that adipose tissue acts as an endocrine and paracrine organ by secreting various proteins (adipokines).1 These bioactive molecules, including leptin, adiponectin, visfatin, omentin, tumor necrosis factor-α, resistin, retinol-binding protein 4 (RBP4), and many others, influence metabolic processes, such as food intake, glucose and lipid metabolism, inflammation, and insulin resistance.2 Adipose tissue is an important inflammatory source in obesity and type 2 diabetes due to the cytokines produced by the adipose tissue as well as macrophages that have infiltrated the adipose tissue during the progression of obesity.3
RBP4 is an adipokine recently discovered to be elevated in insulin-resistant states and obesity in mice4 and humans.3,5 RBP4 is a transport protein for retinol (vitamin A) and is normally bound to transthyretin (TTR) in the circulation. In many studies the level of serum RBP4 elevation correlates highly with the degree of insulin resistance in both mice and humans, and serum RBP4 levels are suggested to be highly predictive of metabolic syndrome risk in a large population-based study.6 However, not all studies agree with these results and find that either serum RBP4 levels are unaltered in insulin resistance/diabetes or that RBP4 mRNA levels in human abdominal subcutaneous tissue levels are even downregulated in obesity.7
Protein tyrosine phosphatase 1B (PTP1B) is a ubiquitously expressed nonreceptor tyrosine phosphatase and a key negative regulator of leptin and insulin signaling.1 High-fat diet feeding in mice has been shown to increase PTP1B levels in a number of tissues, such as the brain, muscle, liver, and adipose tissue,1,8–10 and global PTP1B−/− mice exhibit reduced adiposity and improvements in insulin sensitivity.11,12 However, this improvement in insulin sensitivity is tissue specific as adipose-specific PTP1B−/− mice exhibit an increase in leptin production and mild glucose intolerance.1 Low-grade inflammation, such as that caused by obesity and prolonged high-fat diet feeding, has been shown to lead to an induction of endoplasmic reticulum (ER) stress13–15 and result in an increase in PTP1B protein and mRNA expression levels.10,16 An increase in ER stress response genes, such as binding immunoglobulin protein (BIP), activated transcription factor 4 (ATF4), and glucose-regulated protein 94 (GRP94) in mice is associated with obesity and insulin resistance.
In this study we aimed to investigate if serum and adipose tissue RBP4 protein levels are altered in human obesity and type 2 diabetes in a cohort of men resident in northeast Scotland and if this can be manipulated by either diet or metformin treatment in obese/diabetic subjects. The method of measuring RBP4 levels has been suggested to account for some of the discrepancies between different studies and quantitative western blotting for protein RBP4, and using full-length RBP4 antibody has been suggested as a “gold standard” for RBP4 level determination.17 We therefore used this method to assess both serum and adipose tissue RBP4 protein levels in normal/overweight, obese, and obese/diabetic subjects and correlated these with other markers of adiposity and insulin resistance. In addition, while there is evidence to support that obesity and high-fat diet feeding leads to induction of the ER stress response and induction of the ER stress-response genes in adipose tissue in mice,13 to our knowledge not much data are available with regards to humans. We therefore analyzed PTP1B expression levels and levels of BIP, ATF4, and GRP94 in adipose tissue of normal/overweight subjects and compared it to obese and obese/diabetic subjects in northeast Scotland.
Methods
Volunteers and protocols
Male, nonsmoking subjects were included if they were not on any special diet and had a normal medical examination excluding their diabetic status, where appropriate. Only those diabetic volunteers who were controlling their diabetes by diet or metformin (500 mg four times per day) were accepted. These inclusion criteria were also checked with the participant’s primary-care physician. All patients provided informed written consent before inclusion in the study, which was approved by the North of Scotland Research Ethics Committee.
The study comprised three groups (Table 1): normal/overweight volunteers with BMI < 30 (n = 21), obese volunteers with BMI > 30 (n = 9) and obese/diabetic volunteers with BMI > 30 (n = 14) controlling their diabetes by diet or metformin. All blood samples were taken overnight after 10–12 hours fasting. Each subject’s height, weight, waist, and hip were measured using standard protocols.18 Percentage body fat was determined using air-displacement whole-body plethysmography (BodPod Body Composition System; Life Measurements Instruments, Concord, CT).
Table 1.
Baseline characteristics of the normal/overweight, obese, and diabetic study volunteers. Normal/overweight volunteers have a BMI < 30
| n | Normal/overweight | Obese | Obese/diabetic | P value (ANOVA) |
|---|---|---|---|---|
|
|
||||
| 21 | 9 | 14 | ||
| Age | 46.1 (9.2) | 54.8 (13.8) | 52.8 (13.2) | 0.2 |
| Waist circumference (cm) | 89.6 (5.7) | 113 (6.5) | 120.2 (11.5) | <0.001 |
| Body mass index (kg/m2) | 25.5 (2.10) | 34.3 (5.02) | 36.7 (6.3) | <0.001 |
| Height (m) | 1.76 (0.64) | 1.78 (0.08) | 1.74 (0.66) | 0.351 |
| Body weight (kg) | 79.2 (7.6) | 109 (13.8) | 111 (18.5) | <0.001 |
| Body weight/height ratio | 0.51 (0.04) | 0.64 (0.05) | 0.69 (0.07) | <0.001 |
| Percentage body fat | 22.8 (6.8) | 38.3 (6.1) | 38.2 (7.0) | <0.001 |
| Waist/hip ratio | 0.88 (0.05) | 0.96 (0.07) | 1.05 (0.14) | <0.001 |
| Plasma cholesterol (mmol/L) | 4.73 (1.32) | 5.24 (1.06) | 4.38 (0.49) | 0.17 |
| Plasma HDL cholesterol (mmol/L) | 1.28 (0.33) | 1.15 (0.34) | 1.19 (0.27) | 0.54 |
| Plasma LDL cholesterol (mmol/L) | 3.08 (1.11) | 3.38 (0.80) | 2.54 (0.42) | 0.082 |
| Triglycerides (pmol/L) | 1.09 (0.52) | 1.42 (0.53) | 1.80 (1.08) | 0.04 |
| OGTT plasma glucose 0 hours (mmol/L) | 5.42 (0.74) | 5.88 (0.68) | 8.92 (2.82) | <0.001 |
| OGTT plasma glucose 2 hours (mmol/L) | 5.22 (2.13) | 4.75 (1.3) | 13.8 (3.80) | <0.001 |
| Fasting plasma insulin (mU/I) | 4.74 (2.77) | 9.81 (4.03) | 16.3 (10.6) | <0.001 |
| Fasting plasma leptin (ng/mL) | 4.08 (1.88) | 15.60 (12.34) | 19.44 (8.84) | 0.006 |
| C-reactive peptide (μg/mL) | 1.22 (1.82) | 2.39 (1.50) | 2.78 (2.29) | 0.127 |
| HOMA-IR | 1.19 (0.91) | 2.66 (1.25) | 6.39 (4.24) | <0.001 |
| HOMA beta cell | 52.6 (26.7) | 80.9 (33.2) | 84.6 (60.3) | 0.032 |
| QUICKI | 1.12 (0.22) | 1.44 (0.21) | 1.66 (0.24) | <0.001 |
Notes: Data are mean (standard deviation) for all parameters. P values shown on the right are one-way analysis of variance.
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; OGTT, oral glucose tolerance testing; QUICKI, quantitative insulin-sensitivity check index.
Fat biopsies
A subcutaneous, abdominal region, adipose tissue biopsy was taken after an overnight fast (10–12 hours). The biopsy was performed in the periumbilical triangle with a 14G needle after intradermal anesthesia with 2% lignocaine. Adipose tissue was drawn by successive suctions into a 50 mL syringe. We routinely obtained 0.5–1 g adipose tissue. Tissue was immediately frozen in liquid nitrogen, and samples were subsequently processed for RNA and protein extraction.
Oral glucose tolerance testing
Volunteers fasted overnight (10–12 hours) for oral glucose tolerance testing (OGTT). Blood samples were drawn at 0 and 120 minutes after consuming 75 g of glucose. Plasma glucose levels (and triacylglycerol) were measured using an automated clinical analyzer (Kone Oyj, Espoo, Finland). Plasma insulin was measured using an enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden). The interassay and intraassay coefficients of variation for insulin using this assay were 2.6%–3.6% and 2.8%–3.4%, respectively. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using fasting glucose and insulin values. Total cholesterol, low-density lipoprotein, and high-density lipoprotein cholesterol were analyzed using commercial kits (Microgenics GmbH, Milton Keynes, UK).
Plasma ELISAs
Plasma leptin and C-reactive protein (CRP) were detected, in duplicate, using commercial kits according to the manufacturer’s instructions (R&D Systems, Abingdon, UK). For leptin the minimum level of detection was <7.8 pg/mL and the interassay and intraassay CVs were 4.2% and 3.2%, respectively. For CRP the minimum level of detection was 0.005 ng/mL and the interassay and intraassay CVs were 8.7% and 8.7%, respectively.
Biochemical analyses
Adipose tissue lysates were prepared by extraction in radio immunoprecipitation assay (RIPA) buffer, as described previously.1,19 Ten micrograms of human adipose tissue lysates were used for analysis. Serum RBP4 levels were measured by adding 1 μL of patient’s serum directly into 29 μL of 1% sodium dodecyl sulfate (SDS)-loading buffer and boiling for 10 minutes.20 Proteins were resolved by SDS-polyacrylamide gel electrophoresis (10% gels in 2-(N-morpholino)ethanesulfonic acid buffer), transferred to nitrocellulose membranes and immunoblots performed using polyclonal antibodies against antihuman RBP4 and TTR, following the manufacturer’s instructions. Proteins were visualized using enhanced-chemiluminescence and quantified using a high-sensitivity imaging system (Fusion imaging system and Bio1D software; Vilber Lourmat, Marne-La-Vallée, France).1,10,19,21
mRNA expression analysis
Total RNA was isolated from adipose tissue using TRI Reagent® (Ambion, Warrington, UK) according to the manufacturer’s protocol. First-strand complementary DNA was synthesized from 1 μg of total RNA employing the Biorscript™ Preamplification System (Bioline, London, UK) and an oligo(dT)12–18 primer as the reverse primer. Target genes were then amplified by real-time polymerase chain reaction (PCR) using GoTaqTM qPCR Master Mix (Promega, Southampton, UK) in a Roche LightCycler® 480 System (Roche Diagnostics, Burgess Hill, UK). Relative gene expression was calculated using the comparative Ct (2−ΔΔCt) method, as described previously.10
Statistical analyses
The statistical package GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used for analysis. Results are reported as mean ± standard deviation. One-way analysis of variance (ANOVA) was performed for analysis of more than two variables, followed by a post-hoc test for detection of significance. Simple linear correlation (Pearson’s correlation) was used for quantitative data. Spearman correlation coefficient was used for qualitative data to estimate the association of RBP4 levels and BMI, leptin, insulin, adiponectin, CRP, cholesterol, triglycerides, HOMA-IR, waist/hip ratio, lean weight, fat mass, and percentage fat mass. The P value is significant if P ≤ 0.05.
Results
There were no significant differences in age or height of the volunteers; however they had significantly different waist circumference (ANOVA; P < 0.001), BMI (P < 0.001), percentage body fat (P < 0.001), waist/hip ratio (P < 0.001), and leptin (P < 0.01) (Table 1). In addition, markers of insulin resistance between the three groups were significantly different, namely OGTT at 0 and 2 hours (P < 0.001), fasting plasma glucose and insulin (P < 0.001), HOMA-IR (P < 0.001), and serum triglycerides (P < 0.05).
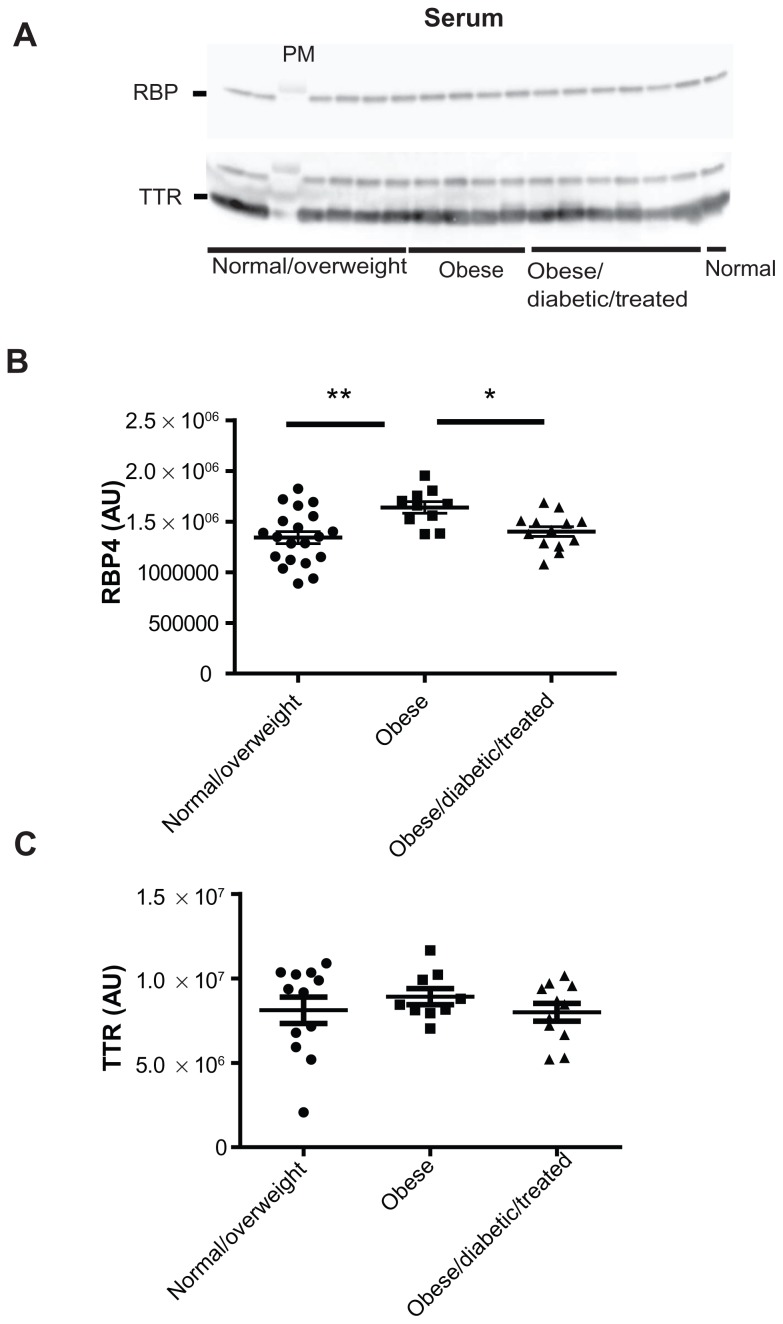
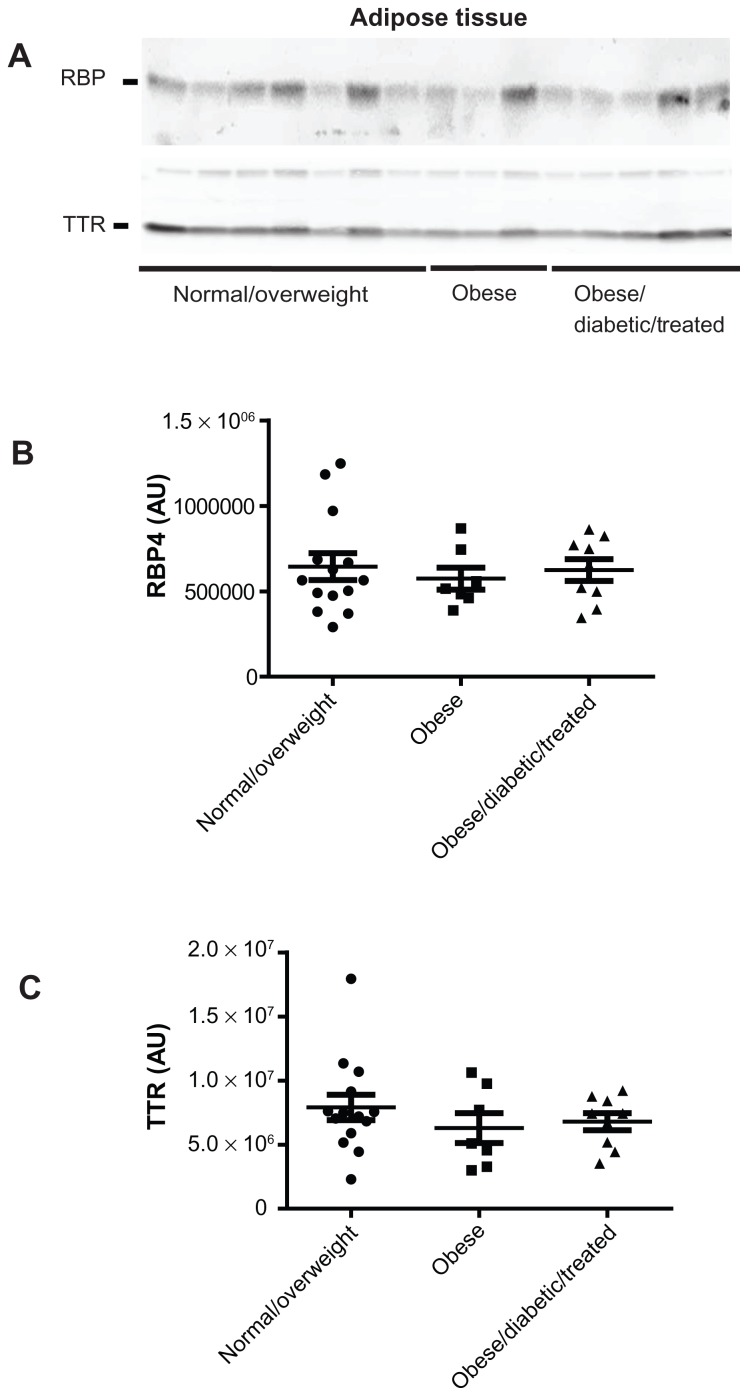
By using anti-human RBP4 and TTR antibodies, we found that obese subjects had significantly higher serum RBP4 protein levels in comparison to the normal/overweight subjects (ANOVA; P < 0.01; treatment effect P = 0.005, F = 6.046) (Figure 1A and B). Furthermore, in obese/diabetic subjects treated with either diet or metformin, serum RBP4 protein levels were completely normalized (ANOVA; P < 0.05) ( normal/overweight 1.34 × 106 ± 0.26 × 106 vs obese 1.64 × 106 ± 0.18 × 106 vs obese/diabetic 1.40 × 106 ± 0.17 × 106), while serum TTR protein levels remained unaltered (Figure 1A and C). Interestingly, while serum RBP4 levels were upregulated in human obesity and normalized by improving whole body insulin sensitivity in obese subjects, the adipose tissue RBP4 protein levels were comparable between all three groups of subjects (Figure 2A and B) as were the serum and adipose tissue TTR levels (Figure 1A and C; Figure 2A and C).
Figure 1.
Serum RBP4 protein levels in normal/overweight, obese, and obese/diabetic subjects treated with diet or metformin. (A) Serum RBP4 and TTR protein levels. One microliter of serum was denatured in SDS-loading buffer and subjected to SDS-PAGE analysis per individual. Quantification of serum (B) RBP4 and (C) TTR protein levels using Bio1D quantitative software. *Denotes P < 0.05; **P < 0.01.
Abbreviations: PAGE, polyacrylamide gel electrophoresis; RBP4, retinol-binding protein 4; SDS, sodium dodecyl sulfate; TTR, transthyretin.
Figure 2.
Adipose tissue RBP4 protein levels in normal/overweight, obese, and obese/diabetic subjects treated with diet or metformin. (A) Adipose tissue RBP4 and TTR protein levels. Ten micrograms of adipose tissue lysate was denatured in SDS-loading buffer and subjected to SDS-PAGE analysis per individual. Quantification of adipose tissue (B) RBP4 and (C) TTR protein levels using Bio1D quantitative software.
Abbreviations: PAGE, polyacrylamide gel electrophoresis; RBP4, retinol-binding protein 4; SDS, sodium dodecyl sulfate; TTR, transthyretin.
To assess if serum and/or adipose RBP4 protein levels correlated with any of the adiposity or insulin sensitivity markers, we performed additional correlation analysis using the Spearman correlation coefficient (R) as done previously.5 While BMI correlated significantly, as expected, with serum leptin (R = 0.89; P < 0.0001), insulin (R = 0.72; P < 0.0001), adiponectin (R = −0.48; P < 0.01), CRP (R = 0.46; P < 0.01), triglycerides (R = 0.36; P < 0.05), and HOMA-IR (R = 0.72; P < 0.0001), we found no correlation between serum and/or adipose RBP4 protein levels and any of the above-mentioned adiposity- and insulin-sensitivity markers (Table 2).
Table 2.
Correlations between plasma RBP4 and BMI, leptin, insulin, adiponectin, CRP, cholesterol, triglycerides, HOMA-IR, waist/hip ratio, lean weight, fat mass, and percentage fat mass
| Clinical features | Normal/overweight n = 12 |
Obese n = 9 |
Obese/diabetic n = 11 |
|||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| r | P | r | P | r | P | |
| Variables | ||||||
| BMI | −0.01 | 0.487 | −0.45 | 0.114 | −0.17 | 0.307 |
| Leptin | −0.43 | 0.107 | −0.2 | 0.307 | 0.23 | 0.251 |
| Insulin | −0.03 | 0.457 | 0.23 | 0.276 | −0.31 | 0.177 |
| Adiponectin | −0.16 | 0.309 | 0.27 | 0.247 | 0.52 | 0.051* |
| CRP | −0.315 | 0.159 | −0.53 | 0.074 | −0.12 | 0.364 |
| Cholesterol | 0.15 | 0.324 | −0.07 | 0.44 | 0.43 | 0.09 |
| Triglycerides | 0.21 | 0.252 | 0.12 | 0.388 | −0.06 | 0.426 |
| HOMA-IR | −0.06 | 0.431 | 0.37 | 0.168 | −0.24 | 0.242 |
| Waist/hip | −0.08 | 0.406 | 0.30 | 0.218 | 0.03 | 0.462 |
| Lean weight | −0.17 | 0.301 | −0.02 | 0.491 | −0.47 | 0.071 |
| Fat mass | −0.07 | 0.414 | 0.07 | 0.44 | 0.07 | 0.416 |
| % fat | −0.01 | 0.483 | −0.19 | 0.307 | 0.19 | 0.290 |
Note: P value is significant if P ≤ 0.05.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance; RBP4, retinol-binding protein 4.
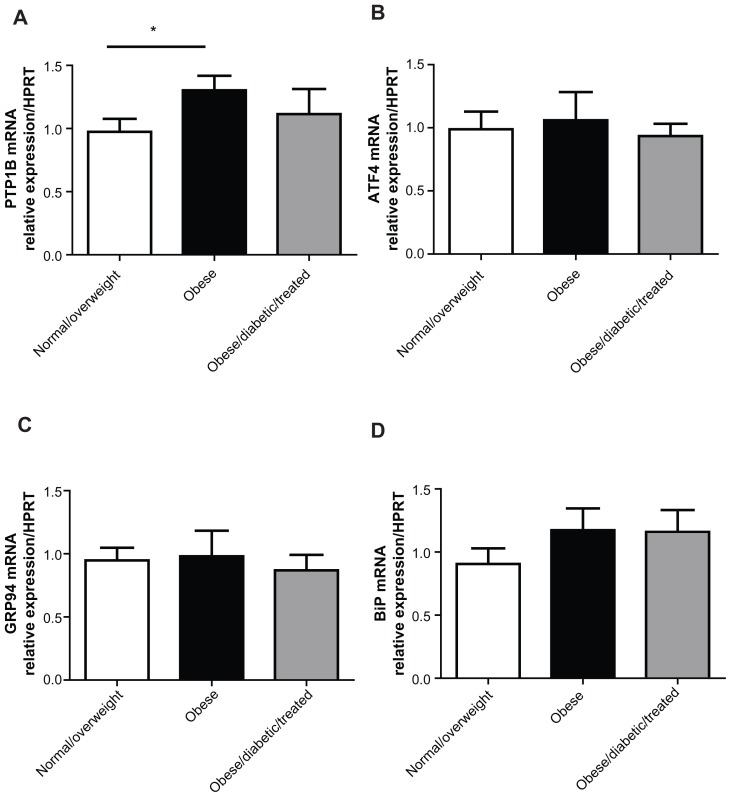
To assess if obesity led to an increase in ER stress-response genes in the adipose tissue of our subjects and if this could be reversed by insulin sensitization, we analyzed adipose tissue gene expression of PTP1B, BIP, ATF4, and GRP94. In agreement with mouse studies, adipose tissue PTP1B gene expression levels were increased in obese subjects in comparison to the normal/overweight group (Figure 3A).1 However, this was not reversed with insulin sensitization in obese/diabetic subjects treated with diet or metformin, suggesting that in humans, as in mice, adipose tissue PTP1B does not regulate insulin sensitivity.1 Gene expression levels of other ER stress-response genes were not regulated by obesity or insulin sensitivity (Figure 3B–D).
Figure 3.
Adipose tissue relative mRNA levels of PTP1B, ATF4, GRP94, and BIP.
Notes: Graphs A–D show relative mRNA levels of the indicated genes in adipose tissue, measured by quantitative real-time PCR and normalized against HPRT mRNA. n = 9 for the normal/overweight group; n = 7 for the obese group; and n = 8 for the obese/diabetic/treated group. Data are represented as mean ± SEM and analyzed using one-way ANOVA with post-hoc analysis. *Denotes P < 0.05.
Abbreviations: ANOVA, analysis of variance; ATF4, activated transcription factor 4; BIP, binding immunoglobulin protein; GRP94, glucose-regulated protein 94; HPRT, hypoxanthine-guanine phosphoribosyltransferase; PCR, polymerase chain reaction; PTP1B, protein tyrosine phosphatase 1B; SEM, standard error of mean.
Discussion
RBP4 is an adipokine proposed to be a marker of human insulin resistance, type 2 diabetes5 and gestational diabetes. RBP4 levels have been found to be normalized by certain antidiabetic drug treatments, such as rosiglitazone or pioglitazone,7 and lowering RBP4 levels has been suggested as a potential antidiabetic therapy. However, many studies have not been able to show this association between human obesity and insulin resistance and have found no correlation between BMI and serum and/or adipose tissue mRNA RBP4 levels.22
In our volunteer study, we demonstrated that serum, but not adipose tissue, RBP4 protein levels are elevated in human obesity. We also showed that this can be completely reversed in obese/diabetic subjects treated with diet or metformin, in agreement with studies from other patient cohorts.5 Thus, improved insulin sensitivity in this small cohort study may be at least partly due to the lowering effects of diet or metformin on serum, but not adipose tissue, RBP4 levels. However, in agreement with other studies that found no correlation between the actual BMI22 and serum RBP4 levels, we also showed that serum and subcutaneous adipose tissue RBP4 protein levels do not correlate with BMI nor do they correlate with any other known investigated adiposity- and insulin-sensitivity markers. This suggests that there may be other factors responsible for the observed rise in serum RBP4 protein levels during the progression of human obesity. Some studies, however, have found an association between serum RBP4 levels and visceral fat,23,24 and it is possible that serum RBP4 levels would correlate directly with this. Since we did not have accurate data on visceral fat for each volunteer, which is normally obtained by magnetic resonance imaging, we cannot exclude the possibility that these would show a correlation.
We also demonstrate that in human subcutaneous adipose tissue, PTP1B expression levels are regulated by obesity in agreement with the mouse studies that demonstrate that adipose tissue PTP1B levels are upregulated upon high-fat diet feeding.1 In agreement with the mouse studies, furthermore, we also observe that an increase in adipose tissue PTP1B levels in human obesity cannot be reversed by improvements in insulin sensitivity in obese/diabetic subjects treated with diet or metformin.
On the other hand, the levels of the other ER stress-response genes remain unaltered in the adipose tissue of the three study groups, suggesting that perhaps the ER stressresponse pathway may not play a major role in this tissue in human obesity. We should, however, be cautious at interpreting these data due to the relatively low sample size that we had available for this specific study; perhaps a larger study size is required to quantitatively examine the role of the ER stress-response pathway in human obesity.
Acknowledgments
We thank Morven Cruickshank and Kim-Marie Moar for their technical support. We are grateful to the Institute of Nutrition and Health, Human Nutrition Unit, for their assistance and to Biomathematics and Statistics Scotland for their assistance in the data analysis.
Funding is provided to MD by an RCUK Fellowship, Diabetes UK, British Heart Foundation, European Association for the Study of Diabetes, Tenovus Scotland, and the Royal Society. NM is funded by an intermediate basic research fellowship from the British Heart Foundation and Tenovus Scotland. NH, MC, and KMM are supported by a grant from the Scottish Government.
Footnotes
References
- 1.Owen C, Czopek A, Agouni A, et al. Adipocyte-specific protein tyrosine phosphatase 1B deletion increases lipogenesis, adipocyte cell size and is a minor regulator of glucose homeostasis. PLoS One. 2012;7(2):e32700. doi: 10.1371/journal.pone.0032700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Esteve E, Ricart W, Fernandez-Real JM. Adipocytokines and insulin resistance: The possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32(Suppl 2):S362–S367. doi: 10.2337/dc09-S340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 5.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354(24):2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 6.Qi Q, Yu Z, Ye X, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab. 2007;92(12):4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 7.McTernan PG, Kumar S. Editorial: Retinol-binding protein 4 and pathogenesis of diabetes. J Clin Endocrinol Metab. 2007;92(7):2430–2432. doi: 10.1210/jc.2007-1054. [DOI] [PubMed] [Google Scholar]
- 8.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283(21):14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A, Uetani N, Simoncic PD, et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2(4):497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 10.Agouni A, Mody N, Owen C, et al. Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically-induced endoplasmic reticulum stress. Biochem J. 2011;438(2):369–378. doi: 10.1042/BJ20110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20(15):5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 14.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Bettaieb A, Liu S, Xi Y, et al. Differential regulation of endoplasmic reticulum stress by protein tyrosine phosphatase 1B and T cell protein tyrosine phosphatase. J Biol Chem. 2011;286(11):9225–9235. doi: 10.1074/jbc.M110.186148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50(4):814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoggard N, Johnstone AM, Faber P, et al. Plasma concentrations of alpha-MSH, AgRP and leptin in lean and obese men and their relationship to differing states of energy balance perturbation. Clin Endocrinol (Oxf) 2004;61(1):31–39. doi: 10.1111/j.1365-2265.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 19.Mody N, Agouni A, McIlroy GD, Platt B, Delibegovic M. Susceptibility to diet-induced obesity and glucose intolerance in the APP (SWE)/PSEN1 (A246E) mouse model of Alzheimer’s disease is associated with increased brain levels of protein tyrosine phosphatase 1B (PTP1B) and retinol-binding protein 4 (RBP4), and basal phosphorylation of S6 ribosomal protein. Diabetologia. 2011;54(8):2143–2151. doi: 10.1007/s00125-011-2160-2. [DOI] [PubMed] [Google Scholar]
- 20.Mody N, Graham TE, Tsuji Y, Yang Q, Kahn BB. Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am J Physiol Endocrinol Metab. 2008;294(4):E785–E793. doi: 10.1152/ajpendo.00521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agouni A, Owen C, Czopek A, Mody N, Delibegovic M. In vivo differential effects of fasting, re-feeding, insulin and insulin stimulation time course on insulin signaling pathway components in peripheral tissues. Biochem Biophys Res Commun. 2010;401(1):104–111. doi: 10.1016/j.bbrc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Yao-Borengasser A, Varma V, Bodles AM, et al. Retinol binding protein 4 expression in humans: Relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92(7):2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino S, Fujiwara M, Suzukawa K, et al. Visceral obesity is associated with the metabolic syndrome and elevated plasma retinol binding protein-4 level in obstructive sleep apnea syndrome. Horm Metab Res. 2009;41(3):221–226. doi: 10.1055/s-0028-1100411. [DOI] [PubMed] [Google Scholar]
- 24.Jia W, Wu H, Bao Y, et al. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab. 2007;92(8):3224–3229. doi: 10.1210/jc.2007-0209. [DOI] [PubMed] [Google Scholar]