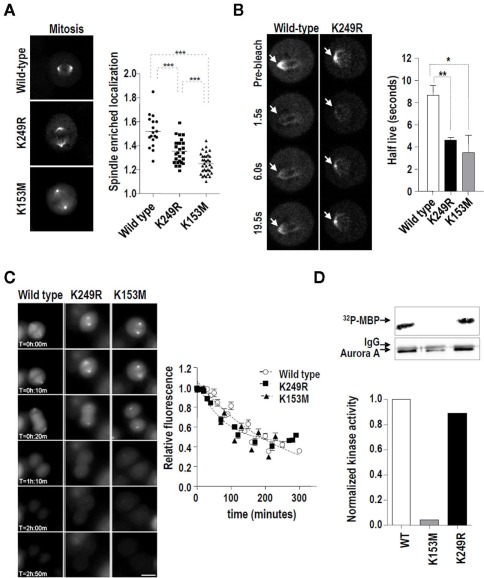
Figure 4.
Lack of SUMOylation at K249 alters the dynamics of Aurora-A at the spindle during mitosis without affecting its kinase activity or stability. (A) EGFP-tagged Aurora-A was visualized in HeLa cells stably expressing the wild type, K153M, or K249R variants. EGFP signals were quantified at the spindle (poles and microtubules) and in the rest of the cell. The graph shows the enrichment of EGFP–Aurora-A signal at the spindle [established as the (spindle EGFP signal)/(remaining cellular EGFP signal) ratio] in the stable cell lines. Pictures show representative metaphases for each EGFP-tagged Aurora-A variant. From three different clones of each variant, a total of 18, 26, and 34 mitoses were detected for the wild type, K249R, and K153M variants, respectively ***p < 0.001. (B) The same HeLa cells were used in a series of fluorescence recovery after photobleaching (FRAP) experiments. Representative images of the recovery of Aurora-AWT and Aurora-AK249R at the centrosomes during mitosis are shown. Arrows indicate the spindles (poles and microtubules) that were measured. The graph shows the half-lives obtained using regression analysis to integrate data from 10 cells each. The half-lives of the EGFP–Aurora-A variants at mitotic centrosomes were compared using an unpaired t-test (*p < 0.05, **p < 0.01). (C) The expression of EGFP–Aurora-A variants in HeLa stable clones was measured using time-lapse microscopy. EGFP signals were quantified for 3 h every 10 min from the first frame in which metaphase was detected. Three clones from each different Aurora-A form were analyzed. Five cells from each clone were followed in this experiment. Representative frames and the quantification of the three different variants are shown. (D) In vitro kinase activity toward recombinant MBP of V5-tagged Aurora-A proteins. HEK293 cells were transiently transfected with the indicated V5–Aurora-A expression vectors and the resulting exogenously expressed proteins were immunoprecipitated with α-V5 antibody. The 32P-MBP signal was quantified considering the total amount of V5–Aurora-A protein in each case.