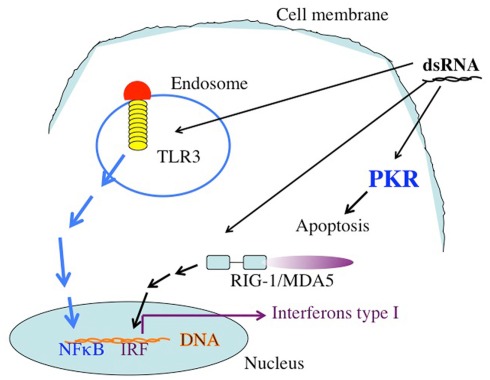
Figure 1.
The targets of dsRNA. TLR3 recognizes dsRNA extacellularly or in endosomal compartments. The TLR3 TIR domain is associated with the adaptor molecule TRIF, which upon ligation activates the protein kinases TBK-1 and IKKe. IRF-3 is phosphorylated by TBK-1 and IKKe on C-terminal serines, leading to its dimerization and translocation into the nucleus. Active IRF-3 induces transcription from the IFN-β promoter. Intracellular dsRNA is recognized by the RNA helicase RIG-I (or MDA5), which activates TBK-1 and IKKe via its CARD domain, leading to IRF-3 activation as well. Both pathways also activate NFκB by mechanisms that are not fully understood yet. Secreted IFN-β binds to the IFN receptor (IFNR) leading to transcriptional activation of ISGs (interferon stimulated genes), such as IRF-7. IRF-7 further stimulates transcription from the IFN-α and -β promoters in a positive feedback loop. In addition to these targets, which are responsible for the activation of the immune system dsRNA binds to the enzyme dsRNA dependent protein kinase, PKR. The activation of PKR leads to the phosphorylation of the eIF2a, leading to the inhibition of protein translation.