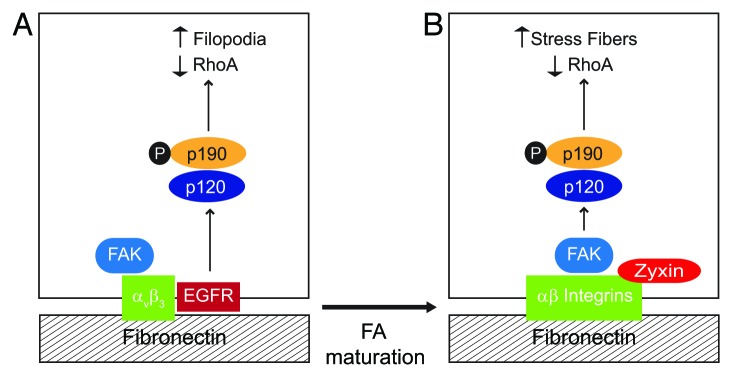
Figure 3. EGFR regulates RhoA-mediated cytoskeletal rearrangements by reconfiguring p190RhoGAP activation. To become fully active the RhoA antagonist p190RhoGAP must be tyrosine phosphorylated by Src kinase, form a complex with p120RasGAP, and undergo recruitment to the cell periphery. Our data suggest that EGFR has an important role in determining the effect of local changes in GTP-Rho activity by regulating p190RhoGAP membrane recruitment. (A) Simplified schematic depicting EGFR-expressing cell adhered to fibronectin. β3 integrin ligation activates FAK and also promotes complex formation with EGFR. β3 integrin-EGFR complexes localize to relatively immature, zyxin-poor FAs. EGFR recruits p120RasGAP which serves as a bridge to p190RasGAP leading to formation of filopodia. (B) EGFR-null cell adhered to fibronectin. RGD-directed αβ integrins activate FAK which serves as a scaffold to recruit p120RasGAP to mature zyxin-positive FAs. p120RasGAP facilitates a bridge between FAK and p190RasGAP leading to formation of actin stress fibers.
