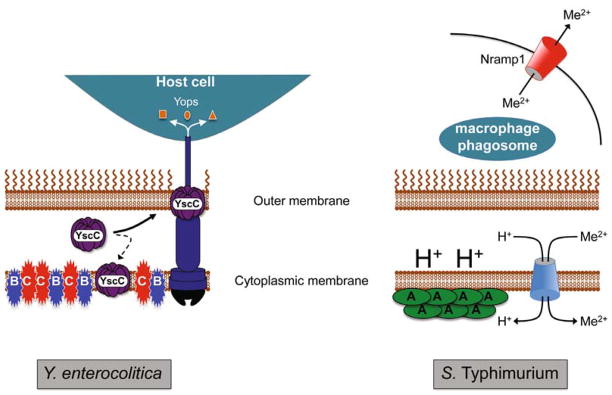
Fig. 2. Summary of the contrasting roles of the Psp system in supporting the virulence of Y. enterocolitica and S. enterica sv. Typhimurium (S. Typhimurium).
In the extracellular pathogen Y. enterocolitica (left) the Ysc T3SS must be produced during host infection to disarm the functions of host immune cells via the function of the exported Yop effector proteins. However, some of the YscC secretin component mislocalizes to the cytoplasmic membrane with the potential to compromise membrane integrity. Secretin mislocalization induces the Psp system and under these conditions the integral cytoplasmic membrane proteins PspB and PspC (but not PspA) are essential for Y. enterocolitica viability. It is not yet known how PspBC prevent or counteract secretin-toxicity. In the intracellular pathogen S. Typhimurium (right) the available divalent cation concentration within the macrophage phagosome is maintained at a low level by the action of the host Nramp1 protein. As a result, S. Typhimurium relies on proton (H+)-dependent and ATP-dependent (not shown) transporters to accumulate divalent cations (Me2+). In this situation, the role of PspA in maintaining the proton motive force is essential because it ensures an energy supply for metal ion import. How PspA achieves this function and the source of the Psp-inducing signal during host infection by S. Typhimurium is unknown.