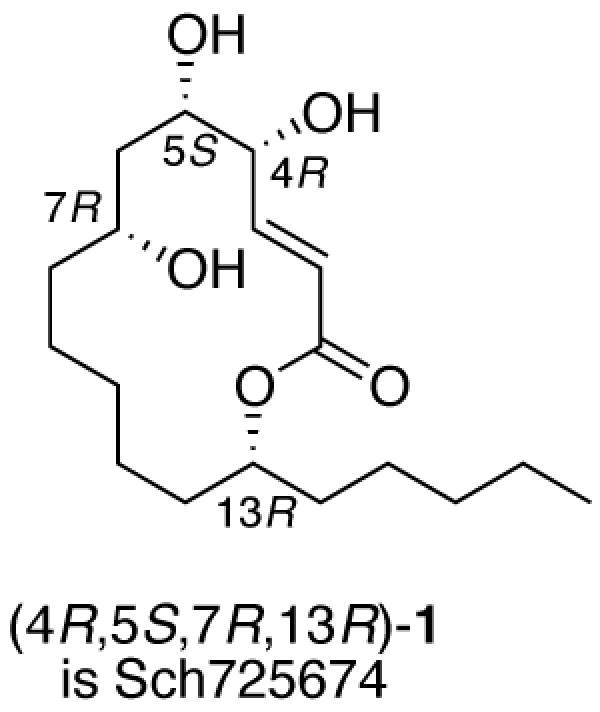
Table 2. Summary of demixing and detagging of lactones M-(R)-15a-h and M-(S)-15a-h to give the 16 final isomers of 1. The assigned structure of Sch725674 is shown as an example.
 | |||
|---|---|---|---|
| isomer of 1 | precursor | amount | yielda |
| (4S,5S,7R,13R) | (R)−15a | 6 mg | 19%b |
| (4S,5S,7S,13R) | (R)−15b | 15 mg | 51% |
| (4R,5R,7R,13R) | (R)−15c | 13 mg | 60% |
| (4R,5R,7S,13R) | (R)−15d | 8 mg | 51% |
| (4S,5S,7R,13S) | (S)−15a | 5 mg | 15%b |
| (4S,5S,7S,13S) | (S)−15b | 17 mg | 67% |
| (4R,5R,7R,13S) | (S)−15c | 3 mg | 14%b |
| (4R,5R,7S,13S) | (S)−15d | 11 mg | 29% |
| (4S,5R,7R,13R) | (R)−15e | 11 mg | 29%b |
| (4S,5R,7S,13R) | (R)−15f | 12 mg | 37%b,c |
| (4R,5S,7R,13R) | (R)−15g | 11 mg | 32%b |
| (4R,5S,7S,13R) | (R)−15h | 5 mg | 40%b |
| (4S,5R,7R,13S) | (S)−15e | 13 mg | 45%b |
| (4S,5R,7S,13S) | (S)−15f | 6 mg | 17%a |
| (4R,5S,7R,13S) | (S)−15g | 14 mg | 73%a |
| (4R,5S,7S,13S) | (S)−15h | 7 mg | 36%a |
includes demixing, detagging and flash chromatography
repurified by chiral chromatography
contains 6% of the C7 epimer
