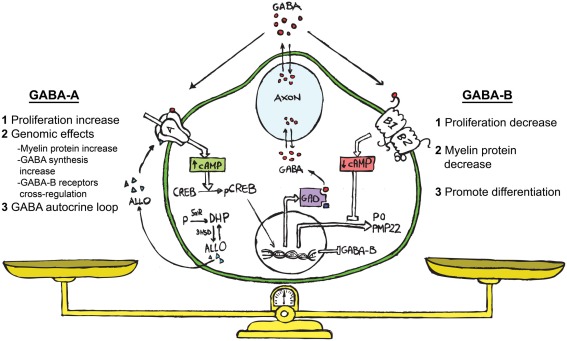
Figure 1.
Schematic representation showing ALLO, GABA and its receptors (GABA-A and GABA-B) acting in the bidirectional cross-talk between neurons and SC. We propose the following hypothesis on the functional role of the GABAergic system in SC. GABA, coming from the neuronal compartment or produced by the SC (Magnaghi et al., 2010), may affect the paracrine cross-talk between these cells. The extracellular GABA might interact with the GABA-B receptor on the SC surface, decreasing their proliferation (Magnaghi et al., 2004). This challenge then diminishes the cAMP levels, reducing the myelin proteins expression (Magnaghi et al., 2004) and prompts the SC to start differentiation. The neurosteroid ALLO, which is produced by SC (Baulieu and Schumacher, 1997; Melcangi et al., 1999b) allosterically activates the GABA-A receptor on the SC surface (Magnaghi et al., 2006). This modulates the expression and the responsiveness of the GABA-B receptor (Magnaghi et al., 2006, 2010), and in turn its desensitization. Contemporarily, ALLO stimulates the SC proliferation (Perego et al., 2011), inducing other genomic effects such as the increase of myelin proteins (Melcangi et al., 1999a; Magnaghi et al., 2001), and the rising of GAD and GABA levels (Magnaghi et al., 2010). The last effect, being a sort of autocrine mechanism, involving enhanced cAMP levels and the PK-A pathway. Collectively, these findings suggest that an autocrine mechanism involving ALLO and GABA is particularly relevant for the control of SC proliferation/differentiation, and may be considered the result of a balanced activation of GABA-A or GABA-B receptors on SC.