Figure 6.
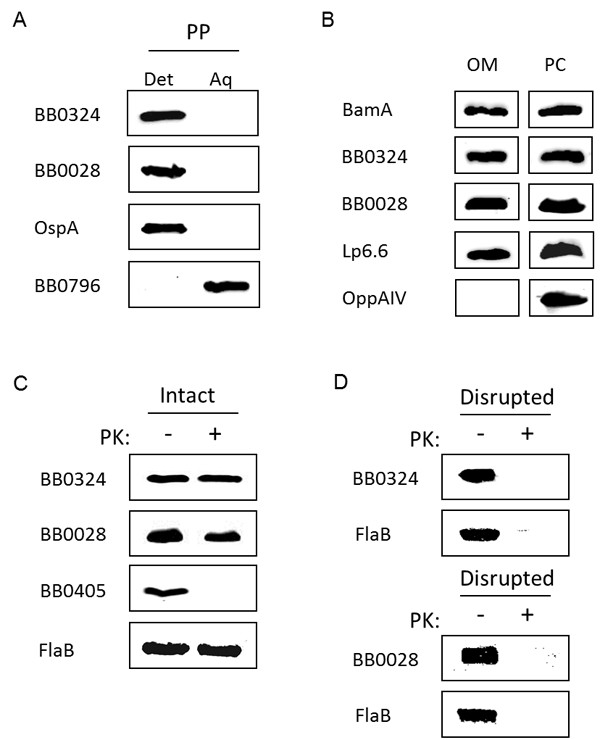
Cellular localization of BB0324 and BB0028. A. BB0324 and BB0028 are integral membrane proteins. Whole-cell lysates of B. burgdorferi B31 were subjected to Triton X-114 phase partitioning, and equal amounts of the detergent-enriched and aqueous phases were subjected to SDS-PAGE and immunoblot analysis with rat anti-BB0324 and rabbit anti-BB0028 antisera. To ensure proper phase separation, a known detergent phase protein and a soluble aqueous phase protein, OspA and BB0796, respectively, were included as controls. B. BB0324 and BB0028 are localized to the B. burgdorferi OM. OM and PC fractions from B. burgdorferi B31-A3-LK cells were isolated as described in Methods. Whole-cell equivalents from each fraction were subjected to SDS-PAGE and immunoblot analysis using BB0324 or BB0028 antisera. For positive controls, fractions were immunoblotted with antibodies against BamA and the known OM lipoprotein Lp6.6, which is anchored to the inner leaflet of the B. burgdorferi OM. To verify OM purity, fractions were also immunoblotted with antibodies against the inner membrane lipoprotein OppAIV. C. BB0324 and BB0028 are subsurface proteins. Whole-cell lysates of B. burgdorferi B31 cells were either mock-treated (-) or proteinase K-treated (+) before being immunoblotted with BB0324 or BB0028 antisera. As a positive control for PK activity, samples were probed with antibodies to BB0405, a known surface-exposed OMP. The mock-treated and the PK-treated samples were also immunoblotted with rabbit anti-FlaB antibodies to ensure equal loading. D. Subsurface BB0324 and BB0028 proteins are degraded by proteinase K. B. burgdorferi cell membranes were disrupted with detergent and lysozyme prior to incubating the lysates in the absence (-) or presence (+) of proteinase K. Samples were immunoblotted using antibodies to BB0324, BB0028, or FlaB (a known periplasmic protein).
