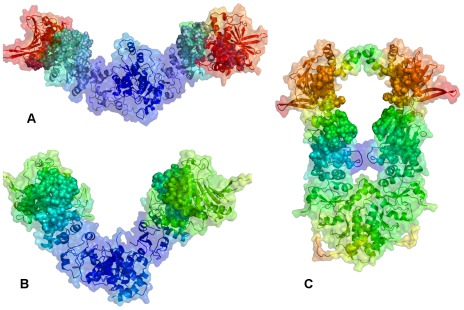
Figure 6. Protein Mobility of the Functional Regions in the HtpG Structures.
The protein mobility of the HtpG structures : (A) extended apo-HtpG solution structure; (B) V-shaped apo-HtpG; and (C) ADP-bound, compact HtpG form. For comparison with the experimental data, the following protein segments monitored in HX-MS experiments [70] were mapped onto the HtpG structures: residues 121–127 (NTD), 192–206 (NTD), 319–334 (M-domain), and 336–359 (M-domain). The Hsp90 structures are depicted in ribbons overlayed with the surface representation at the 50% transparency according to PyMOL. A surface-based representation colored (blue-to-red) according to the protein residue motilities (from more rigid-blue regions to more flexible-red regions). The functional protein segments are shown as spheres with the coloration reflecting changes in their mobility among different structural forms of HtpG.