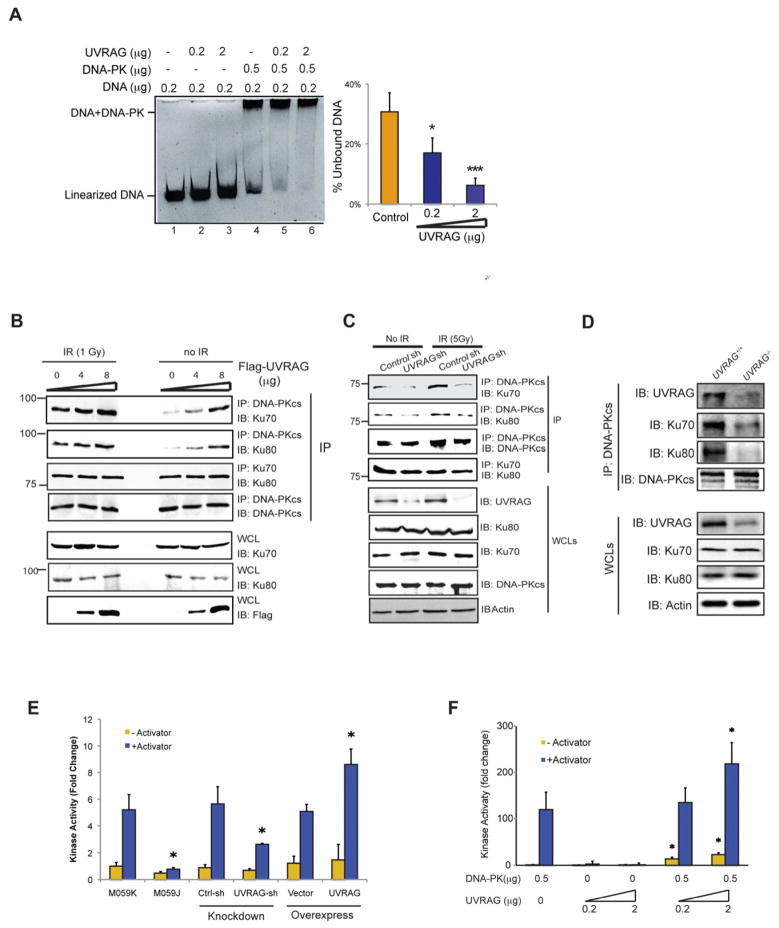
Figure 3. UVRAG Activates DNA-PK During DSB Repair.
(A) UVRAG promotes the DNA end-binding ability of DNA-PK in vitro. Indicated amount of purified UVRAG and DNA-PK complex were mixed with linearized DNA for 1 h, and then subjected to EMSA assay. The DNA end-binding ability was quantified by the percentage of unbound DNA (right). Data represented as mean ± s.d. from three independent experiments. *, P < 0.01; ***, P < 0.001.
(B) Transient expression of UVRAG facilitates the assembly of the DNA-PK complex. 293T cells transfected with increasing amounts of Flag-UVRAG were treated with IR (5 Gy). Co-IP was performed using DNA-PKcs or Ku70 antibody. Western Blot analyzed the amount of DNA-PKcs, Ku70, Ku80, and UVRAG within the complex.
(C) UVRAG knockdown impairs the DNA-PK complex formation. 293T were transfected with control shRNA or UVRAG shRNA for 72 h followed by IR (5 Gy) treatment. WCLs were used for IP with anti-DNA-PKcs followed by IB with the indicated antibody. Bottom panel showed endogenous protein expressions in cells.
(D) DNA-PK complex formation in UVRAG+/+ and UVRAG+/− ES cells. The ES cells were treated with IR (2 Gy). WCLs were used for IP with anti-DNA-PKcs followed by IB with the indicated antibody.
(E) UVRAG enhances DNA-PK activity. DNA-PK activity was measured using the SignaTECT DNA-PK Assay Kit in 293T cell expressing an empty vector, Flag-UVRAG, control shRNA or UVRAG specific shRNA. M059J and M059K cells were used as controls. Data represented as mean ± s.d. from three independent experiments. *, P < 0.05.
(F) in vitro kinase assay with purified UVRAG and DNA-PK complex was performed. Data represented as mean ± s.d. from three independent experiments. *, P < 0.05.