Figure 2. Characterization of human CLR and RAMP1 antibodies by Western blot analysis.
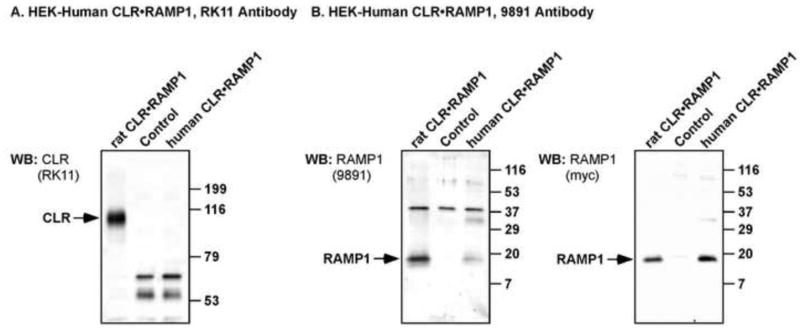
HEK cells were transfected with empty vector (control) or human FLAG-CLR and human myc-RAMP1 and the specificity of anti-rat CLR (RK11) and RAMP1 (9891) antibodies determined by Western blotting. (A) RK11 antibody readily detected rat CLR (positive control, ~96 kDa) but did not cross-react with human CLR. (B) 9891 antibody readily detected RAMP1 from both rat and human (~17 kDa), with no RAMP1 staining detected in vector-transfected control cells. After stripping, blots were re-probed with anti-c-Myc antibody to detect the extracellular epitope tag of rat and human RAMP1. Immunoreactive bands were detected at the same molecular mass for rat and human RAMP1 by anti-c-Myc, indicating specificity of the proteins detected by 9891. As predicted, neither anti-c-Myc nor 9891 detected any immunoreactive proteins of the predicted size of RAMP1 in vector transfected control cells. There were faint signals for human RAMP1 (~35 kDa) with both 9891 and c-Myc antibodies, which we predict to be dimers of RAMP1.
