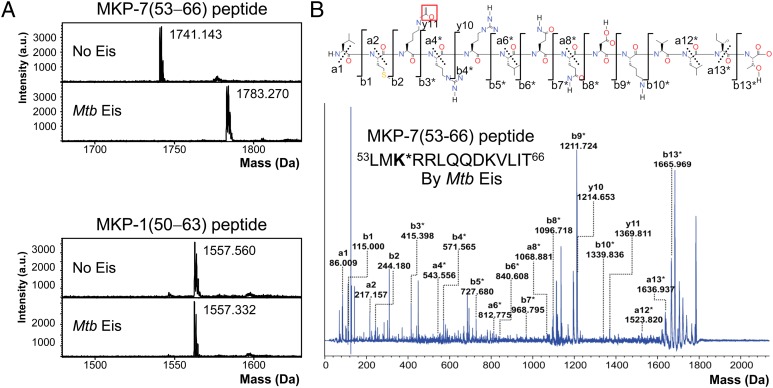
Fig. 1.
Acetyltransferase assay of Mtb Eis using MKP-7(53−66) and MKP-1(50−63) peptides by mass spectrometry. (A) Mass spectra of the MKP-7(53−66) peptide before and after acetylation reaction by Mtb Eis (Upper). The observed increase in the peptide mass by 42 Da indicates that the peptide is acetylated at a single site. Mass spectra of the MKP-1(50−63) peptide before and after acetylation reaction by Mtb Eis (Lower) indicate that this peptide is not acetylated by Mtb Eis. (B) MALDI MS/MS spectrum of the MKP-7(53−66) peptide acetylated by Mtb Eis. The fragments marked with an asterisk (*) are +42 Da-shifted ions, compared with the counterparts that would be generated from the unmodified peptide. The acetylated MKP-7(53−66) fragmentation notation using the scheme of Roepstorff and Fohlman (45) is given above the spectrum. The acetyl group of modified Lys55 is highlighted by enclosing in a red box.