Abstract
This paper provides a brief overview of how we got involved in fMRI work and of our efforts to elucidate the mechanisms underlying BOLD signal changes. The phenomenon discussed here in particular is the post-stimulus undershoot (PSU), the interpretation of which has captivated many fMRI scientists and is still under debate to date. This controversy is caused both by the convoluted physiological origin of BOLD effect, which allows many possible explanations, and the lack of comprehensive data in the early years. BOLD effects reflect changes in cerebral blood flow (CBF), volume (CBV), metabolic rate of oxygen (CMRO2), and hematocrit fraction (Hct). However, the size of such effects is modulated by vascular origin such as intravascular, extravascular, macro and microvascular, venular and capillary, the relative contributions of which depend not only on the spatial resolution of the measurements, but also on stimulus duration, on magnetic field strength and on whether spin echo (SE) or gradient echo (GRE) detection is used. The two most dominant explanations of the PSU have been delayed vascular compliance (first venular, later arteriolar, and recently capillary) and sustained increases in CMRO2, while post-activation reduction in CBF is a distant third. MRI has the capability to independently measure CBF and arteriolar, venous, and total CBV contributions in humans and animals, which has been of great assistance in improving the understanding of BOLD phenomena. Using currently available MRI and optical data, we conclude that the predominant PSU origin is a sustained increase in CMRO2. However, some contributions from delayed vascular compliance are likely, and small CBF undershoot contributions that are difficult to detect with current arterial spin labeling technology can also not be excluded. The relative contribution of these different processes, which are not mutually exclusive and can act together, is likely to vary with stimulus duration and type.
Keywords: BOLD, undershoot, CBF, CBV, CMRO2, blood flow, blood volume, oxygen metabolism, hematocrit, fMRI, vascular compliance, venule, arteriole, capillary
In view of the historical nature of this special Neuroimage issue, this review starts with a personal reflection by the first author, followed by a comprehensive overview of current literature as interpreted by all authors.
History and Motivation (by first author)
My interest in fMRI was relatively late, well beyond the exciting first years of most BOLD discoveries. This was based in part on my attention to developments in diffusion tensor MRI and my continuing interest in spectroscopy, as well as on some skepticism that I felt toward the fMRI field, a new phenomenon that did not seem easy to interpret and was prone to many artifacts. It was not until June of 1995 that Risto Kauppinen convinced me to perform some combined high-sensitivity 4.7T fMRI/MRS experiment to determine whether lactate was really being produced during prolonged visual stimulation in the cat brain. Despite having a 70s time resolution for MRS and the cat having a huge visual cortex with a burgeoning spectroscopic SNR, we found no lactate changes (Kauppinen et al., 1997). This convinced us that early suggestions regarding anaerobic metabolism during increased brain functioning might be incorrect and provided an early indication that fMRI signal changes were a mystery worthwhile to be investigated. A second interesting phenomenon was that most investigators interested in elucidating the BOLD mechanism only used extravascular BOLD theories, while there were some clear indications that much of the effect was intravascular, especially at 1.5 Tesla (Lai et al., 1993). Third, most models contained adjustable constants (A, K, beta, alpha), some without a particularly clear physiological meaning. This provided sufficient motivation to start working on a detailed physiological and physical theory. Together with Risto’s student Joni Oja, I spent about 6 months reading up on physiology and deriving equations and we were able to come up with a reasonably plausible 4-compartment (tissue, arterioles, capillaries and venules) theory for parenchymal spin echo effects (van Zijl et al., 1998). The model could satisfactorily explain hypoxic BOLD effects and, assuming predominantly intravascular SE effects, could even be used to determine plausible absolute CBV values using endogenous deoxyhemoglobin as the natural contrast agent. Based on Fick’s principle, we derived an exact expression for the fraction of venous deoxyhemoglobin (dHb) versus total hemoglobin (Hbtot, proportional to Hct), that contained no unclear constants and, contrary to then-existing theories, was also valid for hypoxia (van Zijl et al., 1998):
| [1] |
in which
| [2] |
Ca is arterial oxygen content and OEF the oxygen extraction fraction; Ya and Yv are the arterial and venous oxygen saturation fractions, respectively. This expression was combined with a dependence of the intravascular relaxation rate R2 on the deoxyhemoglobin fraction to describe SE effects. We used exchange theory to describe the relaxation, but it would work just as well with diffusion theory (similar curves as a function of echo time). We (Oja et al., 1999), in line with earlier work (Kennan et al., 1994) and recent data (Jochimsen et al., 2004) also showed that fMRI spin echo effects at 1.5T were dominated by intravascular effects (probably in draining veins), contrary to the assumption of others that microvessels dominate SE effects as based on susceptibility theory for extravascular BOLD effects. Excellent theories for such extravascular effects had already been described by several groups (Boxerman et al., 1995; Ogawa et al., 1993; Weisskoff et al., 1994; Yablonskiy and Haacke, 1994) and extended by others (Jensen et al., 2001; Kiselev and Posse, 1999). With respect to physiology, the BOLD extravascular effects around the vasculature add as an extra term into the effective transverse relaxation rate:
| [3] |
in which Δχdeoxy is the magnetic susceptibility effect for fully deoxygenated blood (reported to range between 0.18 and 0.35ppm), fi the vessel fraction, and CBVi the blood volume for a particular vessel. Thus arterial vessels will hardly contribute (Ya≈1), while venous vessels (Yv≈0.61) dominate the BOLD effect. Of course the situation is more complicated because capillaries also contain deoxygenated blood, but with an oxygenation fraction (Yc) that decreases from 0.98 or lower in the post-arterioles to about 0.61 in the early venules. In addition, Eq. [3] is based on a static dephasing regime, which will not apply in the capillaries (Kiselev and Posse, 1999; Ogawa et al., 1993; Weisskoff et al., 1994; Yablonskiy and Haacke, 1994). Therefore only vascular multi-compartment models (Buxton et al., 2004; Lu et al., 2004a; Uludag et al., 2009; van Zijl et al., 1998) will ultimately provide a complete description, but Eqs. [1–3] are useful for qualitative explanation of intravascular and extravascular BOLD phenomena, especially for the large voxels used in typical fMRI experiments at 1.5T and 3T. The early efforts by many groups in the nineties provided useful working equations, but many questions remained and the investigation of the BOLD mechanism became a main effort in my lab.
The early years of the post-stimulus undershoot (PSU)
Of course we were not the first to notice or become interested in the undershoot. Actually, some of the very first fMRI experiments (Kwong et al., 1992) already showed its existence and the discussion became especially interesting when Jens Frahm and colleagues performed FLASH experiments and showed very reproducible and large undershoots (Frahm et al., 1996; Kruger et al., 1996). The explanation they suggested for this apparent increase in deoxygenated hemoglobin was a continued increase in CMRO2 after the stimulus had subsided, which amounts to an uncoupling of the metabolic and blood flow responses. Around the same time, Buxton et al. (Buxton et al., 1998) were developing the so-called “balloon” model to describe the BOLD signal changes in terms of an oxygen-limited tight coupling between CBF and CMRO2, and explained the undershoot based on a vascular phenomenon (venous ballooning). Also in 1998, the MGH group (Mandeville et al., 1998) reported forepaw stimulation results in rats that showed clear delayed post-stimulus signal recovery in CBV time courses obtained using a long-lived blood contrast agent (MION). Following additional experiments, they developed the windkessel model (Mandeville et al., 1999b), explaining the PSU in terms of delayed venous compliance and especially ruling out continued increases in oxygen metabolism (Mandeville et al., 1999a). Data from one of these experiments is shown in Fig. 1a. A delayed return in CBV after the stimulus seems clear, but, interestingly, the increase in CBV is also delayed while BOLD and CBF increase rapidly. Others soon joined the discussions (Jones et al., 1998; Jones, 1999). These intriguing early experiments and the discrepant interpretations between the Frahm and MGH groups set the stage for a still ongoing discussion of the origin of the undershoot in particular, but also of the correctness of the proposed BOLD models. In this brief review we will focus mainly on the undershoot. Obviously, BOLD studies by themselves could not provide answers to which factors contribute as they depend on the many contributions mentioned above. To acquire a better understanding, it would be needed to develop CBV measurements that could be done easily and repeatedly in humans (no anesthesia) and could be combined with available CBF technology. Preferably, a separation of CBV effects in different vessels, such as possible with optical measurements, should also be available. In the following, we describe some of the MRI developments that occurred since the late nineties that include the use of CBV-sensitive measurements that do not require contrast agents in humans (Lu et al., 2003; Stefanovic and Pike, 2005) and the elucidation of CBV effects in different types of vascular compartments using high resolution MRI in animals (Jin and Kim, 2008b, 2008a; Yacoub et al., 2006). This will be analyzed together with vascular-tree-specific optical data that provide information on the relative contributions from different types of blood vessels to vasodilation during functional activation (Fernandez-Klett et al., 2010; Hillman et al., 2007; Silva et al., 2007). Interestingly, these recent developments have shed some more light on the PSU but have also induced more discussion. While performing such analyses, it is important to keep in mind the specific features (strengths and weaknesses) of each of the methodologies. For instance, MRI signal reflects water in a voxel and has the many complex contributions outlined above, while optical signals (especially in humans, but less so in animals) can be hampered by limited depth penetration and looking at vessels that are not typical for deep parenchyma where the neuronal activation occurs.
Figure 1.
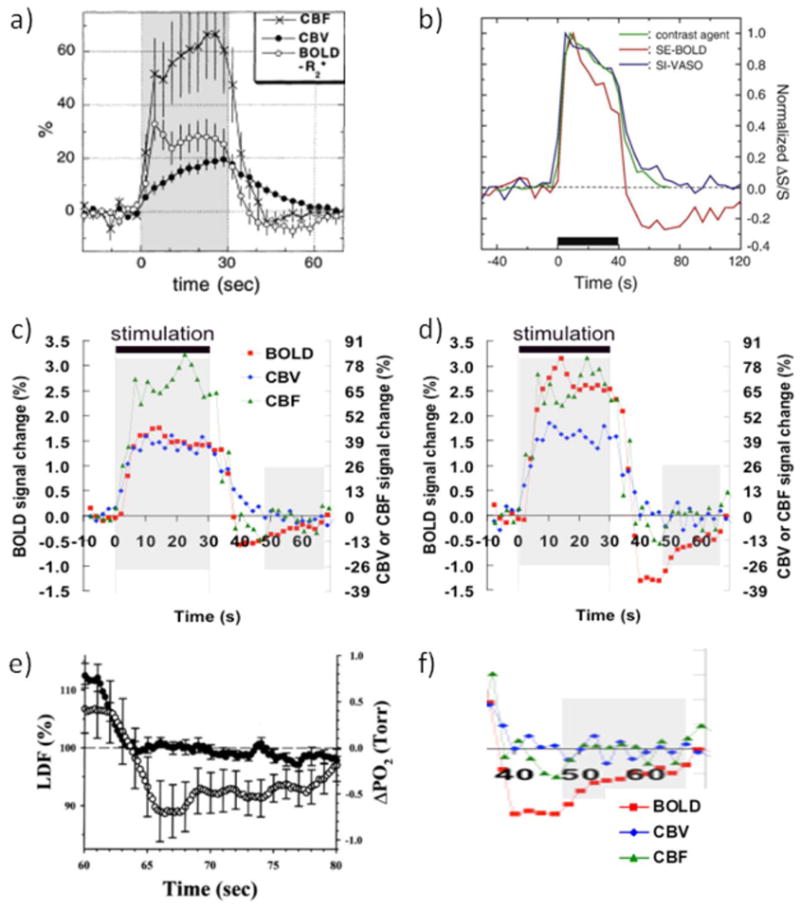
(a-d) Collection of hemodynamic response data illustrating the issues surrounding the BOLD-PSU controversy. (a) GRE-BOLD, CBF and MION-CBV data from forepaw stimulation in alpha-chloralose anesthetized rats, showing delayed vascular compliance following stimulus cessation. However, also note the delayed CBV increase during stimulus onset. Reprinted by permission Reprinted from Mandeville et al., 1999a, Magn Reson Med. 42, 944, with permission from John Wiley and Sons (b) MION-CBV and VASO-CBV deep-cortex data from visual stimulation in isoflurane-anesthetized rats, showing excellent correspondence between the two methods and a rapid response to stimulus onset as well as stimulus cessation. A slower second CBV phase of much smaller magnitude than the positive response can also be seen, which returns to baseline well before the spin echo BOLD-PSU has ceased to exist. Reprinted from Jin and Kim, 2008a, Neuroimage. 40, 59 with permission from Elsevier; (c,d) Average time courses (n = 8) from human visual stimulation (yellow-blue flashing checkerboard) using VASO-CBV, CBF and GRE-BOLD for all voxels activated within a certain approach (c) and for only voxels activated in all three approaches (d), the latter reflecting better localization in parenchymal regions. Notice the lack of significant CBV and CBF delays during the large and prolonged BOLD-PSU in (d). Reprinted from Lu et al, 2004a, J. Cereb. Blood Flow Metab. 24, 764, with permission from Macmillan Publishers Ltd. (e) Laser Doppler flow and oxygen tension changes following 60 s of forepaw stimulation in halothane-anesthetized rats. Notice the lack of a flow undershoot and the prolonged undershoot in oxygen tenson of the same shape as the one found following visual stimulation in humans (d), which is show expanded in (f). Data in (e) were reprinted from Ances et al., 2008b, Neurosci Lett. 306, 106, with permission from Elsevier.
PSUs, big and small
At the end of the nineties, most human scanners were at 1.5 Tesla, where intravascular BOLD effects dominated especially SE, but also GRE effects. I therefore asked the next graduate student (Hanzhang Lu) to study the BOLD effect in humans and to especially account properly for the different contributions of intra- and extravascular effects. Surprisingly, when trying to remove the intravascular effect using inversion recovery based blood signal nulling, Hanzhang did not find any positive (extravascular) BOLD effects, but instead a slight signal reduction that was even more amplified at short TE. This serendipitous discovery led to the development of the vascular space occupancy (VASO) method, allowing us to determine CBV changes without contrast agents (Lu et al., 2003; Lu and van Zijl, 2011). Thus, we could now noninvasively and repeatedly investigate, in the same person, CBF (from arterial spin labeling), CBV and BOLD effects during visual stimulation, which we intended to use for measuring OEF and CMRO2 and to increase our understanding of the BOLD mechanism. While originally not the goal, it was this experiment that spurred our interest in the BOLD PSU. As expected, our consecutive arterial spin labeling (ASL), VASO and BOLD experiments provided detailed hemodynamic responses (Fig. 1c) for the activated regions. However, we were worried that it might not be proper to compare these responses because the accompanying activation maps showed the typical much larger spatial spread for BOLD than for VASO and ASL. We therefore decided that the correct approach would be to compare only voxels that were activated in all three methods. Surprisingly, this spatially restricted analysis showed a large increase in both the positive and negative (PSU) BOLD responses, as illustrated in Fig. 1d. We had previously shown that VASO signals were more localized in the parenchyma while others had suggested a more arteriolar/capillary location for ASL. So we concluded that the BOLD hemodynamic response was much larger in the parenchyma than in the draining veins, where some dilution with blood from non-activated areas probably occurred. Interestingly, these well-localized responses showed a prolonged PSU, during a time period in which CBV and CBF changes were negligible. Based on the BOLD equations, the only possible explanation we could logically come up with was a continued increase in CMRO2 during a period where CBF and CBV had already returned to baseline. This was of course very controversial because it basically refuted the balloon and windkessel models, which not only assumed a tight coupling between oxygen metabolism and CBF but also explained the PSU in terms of delayed venous vascular compliance. When we presented this at the ISMRM in 2004 it led to some interesting discussions and the audience was separated in excited believers and not-so-happy skeptics who felt the data had to be wrong. Of course data are what you measure and thus by definition correct (this applies to the Mandeville data as well as our data), but the experimental conditions under which they are obtained may differ between groups and the interpretation will depend on assumptions made. Submitting the paper also proved difficult, with typical critiques being that the VASO method had not been validated and that flow and metabolism just had to be coupled. In addition we should really explain why the animal experiments showed the slow CBV recovery, even though of course these were not our experiments. Fortunately, we were able to find great supporting evidence in the literature. First of all, breakthrough papers were published (Attwell and Laughlin, 2001; Attwell and Iadecola, 2002) indicating that the large flow increase upon neuronal stimulation was not necessarily metabolically driven but could be a consequence of neuronal signaling. In addition, an article (Ances et al., 2001) reporting on changes in tissue oxygen partial pressure (pO2) and cerebral blood flow (measured by Laser Doppler Flowmetry, LDF) before, during, and after prolonged (60 s) forepaw stimulation in rats, showed a prominent reduction in pO2 while CBF had returned to baseline. Interestingly, the shape of the pO2 curve (Fig. 1e) in this report resembled closely our focal BOLD undershoot (enlarged in Fig. 1f). The time scale of these curves also matched well with observations in cultured neuronal tissues that lengthy periods (30–40s or more) are needed for restoration of ionic gradients after neuronal activity is stopped (Brockhaus et al., 1993; Koch and Barish, 1994). Concomitant to the ionic recovery, the Brockhaus paper showed strong post-stimulus pO2 reductions that increased with stimulus duration (Fig. 8 in Brockhaus). Koch and Barish used 30 s of stimulation, the same length as the data in Figs. 1c-d. They reported an ionic restoration half-life time of 8.3 seconds, which was in good agreement with our PSU and the CMRO2 and OEF recovery curves calculated from that (Lu et al., 2004b). We therefore interpreted our data as reflecting that the increased metabolism needed to reverse ions across the membrane is the major component of brain energy use, in line with Attwell and Laughlin’s calculations. Another potential contributor requiring extra energy would be neurotransmitter resynthesis/recycling. Our paper was finally accepted (Lu et al., 2004b), but many MRI experts demanded more evidence, such as reconciliation between animal and human experiments and validation of the VASO method, all reasonable requests.
VASO versus agent-based CBV experiments
The discrepancy between MION data in animals and the VASO data in humans was a major stumbling block for MRI investigators to accept our results. Fortunately for us, the group of Seong-Gi Kim performed some MION-CBV and VASO experiments during visual stimulation in the cat brain (Jin and Kim, 2008a) that conclusively showed the predominant spatial localization of VASO signal changes to be in the deep cortex (layer IV), similar to MION. Furthermore, the MION and VASO time courses were basically the same within error (Fig. 1b) and showed a somewhat slow return to baseline after stimulation, but much faster than the SE-BOLD PSU in the same middle cortical ROI (Jin and Kim, 2008b). In addition, the rise of both the VASO and MION CBV responses of this study was within the first time point (temporal resolution of 5 s), compared to the very slow rise over a period of 30 s in the Mandeville study (Fig. 1a). When comparing the Kim MION-CBV work with that of Mandeville, several differences need to be kept in mind. First of all, the stimulated regions were visual in cat versus sensory (forepaw) in rat. Secondly the anesthesia was different (isoflurane versus alpha-chloralose) and finally, the field strengths (9.4T versus 4.7T), spatial resolution (0.2 mm3 versus 0.6 mm3), and MRI acquisitions (SE versus GRE) were different. At first glance, differences in field strength, spatial resolution, species or type of neuronal activation seemed unlikely to have a major impact on initial CBV dynamics, so by lack of a better explanation, the different anesthetic appeared to the most plausible cause at the time. However, a follow-up paper on visual stimulation in awake macaques (Leite et al., 2002) confirmed the slow hemodynamic rise and decay during MION-based CBV experiments, disproving this explanation. In addition, optical experiments in both anesthetized (urethane) and awake rats during Whisker stimulation (Martin et al., 2006) showed a rapid rise and return to baseline for total hemoglobin, taken as a measure of CBV. Anesthetized animals showed a slightly delayed response decay, but still much faster than the MION curves in the rat and monkey studies. In view of this, we conclude that the apparently different results between the different MRI-CBV methods and between MRI-MION and optical data is more a reflection of the type of MRI and optical acquisitions and the related mechanisms of contrast than of physiology. This is not unexpected because the MION-CBV contrast mechanism is predominantly susceptibility based. Thus, similar to BOLD, it will depend on vascular size and orientation, intravascular versus extravascular contributions and the use of spin echo versus gradient echo acquisition, which will have different spatial specificity. The relative magnitude of such contributions will vary with field strength and echo time. Some insight can be acquired from the relaxation equation for extravascular susceptibility effects in the static dephasing regime (Eq. [3]), but now for plasma contrast agents:
| [4] |
in which Δχagent depends on the concentration of MION in plasma. Contrary to BOLD, which is focused on (partially) deoxygenated blood, all vessel types contribute for MION. Even in the most simple case of a very high agent concentration that diminishes all blood signals because of a reduction in T2* and T2 of the blood, the agent’s signal effect is complex in that it will include spin density effects (similar to VASO) plus the extravascular effects of equations [3,4] plus contributions from susceptibility effects around vessels smaller than 10μm. The situation is even more complicated than for BOLD susceptibility effects because the BOLD effect itself will interfere with the MION hemodynamic response (Kennan et al., 1998; Kim et al., 2007; Lu et al., 2007). At very short TE it should be equal to VASO.
Since our original study in 2004, others have confirmed the rapid CBV changes in humans. Using visual stimulation combined with hypoxic hypoxia (Tuunanen et al., 2006), it was shown that the BOLD PSU continued much longer than CBV as measured by the VASO technology. Interestingly, while the positive BOLD effect was reduced during hypoxia, hypoxia did not affect the length and magnitude of the PSU. It was concluded that the PSU is not affected by curtailed oxygen availability. Frahm and co-workers recently performed a beautiful study using dynamic T1-weighted 3D MRI to monitor the temporal profile of CBV-related signal changes after administration of the “long-lived” (~ 1hr) blood-pool contrast agent vasovist (Dechent et al., 2011) that binds to albumin. It causes T1 as well as T2 and T2* shortening, but when used at short TE at 3T, the T1 effects can be made to dominate. Their data clearly demonstrated a rapid return of CBV-based changes to baseline after visual stimulation.
Based on the above, we can draw several conclusions for VASO and agent-based CBV studies. First, VASO and spin echo MION CBV data respond similarly to visual stimulation in cats, validating the microvascular CBV origin of the VASO contrast. Microvascular localization by VASO-fMRI is a bit confusing, because a single VASO MRI experiment will darken all blood, thus giving total microvascular plus macrovascular blood volume. However, a functional task elicits microvascular dilatation or restriction and the VASO-fMRI signal changes are restricted to effects from these vessels. This is a bit similar to diffusion imaging, where microscopic effects can be measured with a macroscopic technique. The localization of the VASO signal changes is also clear from human data at lower fields (1.5T and 3T) where BOLD signals include large draining veins, while VASO data display effects only within tissue (Lu and van Zijl, 2005). Note that small errors in nulling will affect the magnitude of the VASO change, but not the microvascular localization.
A second conclusion is that hemodynamic time courses for VASO and a long-lived T1-agent studied in humans also correspond in showing a fast rise and decay with stimulus onset and cessation, respectively. Thus, it appears that much of the controversy regarding earlier interpretations of the BOLD-PSU origin was more a consequence of different MRI-based CBV methods having different spatial specificity than a reflection of physiology. In recent years, more information regarding vascular responses in venules, capillaries and arterioles has become available, which is bringing us closer to solving the puzzle.
Compartmentation of BOLD and vascular effects
In order to shed more light on the PSU-origin controversy, another group (Yacoub et al., 2006) performed very high resolution (0.045 mm3) visual stimulation studies in the cat brain at 9.4T and compared GRE and SE BOLD and GRE-based MION-CBV effects in deep cortex (which they named “tissue”) and closer to the cortical surface (called “vessel”). Since no CBV effects are expected inside large vessels, we use “cortical surface” terminology to reflect that their “vessel” area is still parenchyma but containing pial vessels. The average data over 4 animals showed no detectable difference between GRE BOLD effects in deep cortex and the cortical surface, both displaying a PSU of about 10 s after a stimulus of 20 s. Interestingly, the time course of signal return to baseline after stimulus at the cortical surface was indistinguishable for GRE-CBV and GRE-BOLD (before PSU onset), while the CBV return in deep cortex was delayed. Due to the large variation in the CBV data, an exact time point for return was hard to discern, but probably between 5 and 10 s. This is still comparable within error to the data of Jin and Kim in Figure 1b, which show a clear BOLD undershoot in deep cortex long after CBV with both MION and VASO have returned to baseline. The Yacoub cortical surface data and the Kim deep cortex data therefore convincingly show that CBV alone cannot explain the PSU, while data from deep cortex from both Kim and Yacoub indicate there may be some CBV contribution there. The conclusion of Yacoub et al. was that the effect of vessels other than venous should be assessed. Around the same time, other investigators (Behzadi and Liu, 2005) suggested an arterial compliance model to explain the MION-CBV time courses, which is also expected theoretically as MION-based susceptibility changes affect all vessel types (Eq. [4]).
More insight into the relative contributions of different vasculature comes from recent optical work showing that blood volume changes during neuronal stimulation in pial arteries and arterioles are much larger than in capillaries, which are much larger than in venules (Hillman et al., 2007). This is in line with current understanding that astrocytes play a main role in such control for penetrating arterioles and capillaries, with further upstream dilatation probably being a consequence of cellular signaling (Attwell et al., 2010; Iadecola and Nedergaard, 2007; Koehler et al., 2009; Lauritzen, 2005). When corrected for the fact that venular and capillary volumes are much larger than arteriolar volume (Hillman et al., 2007), the relative contributions to total volume change were found to be about 40/40/20 for arteriole/capillary/venule, respectively (Fig. 2a). Thus, arterioles and capillaries will dominate the MION-CBV experiments. Interestingly, the optical data for capillaries show a delayed return to baseline compared to arteriolar and venular hemodynamic responses (Fig. 2a), which may indicate that the delays found by Yacoub and Jin & Kim are of capillary origin. Recent work using two-photon laser scanning microscopy (Stefanovic et al., 2008) also confirms that the capillaries contribute significantly to total CBV changes during activation.
Figure 2.
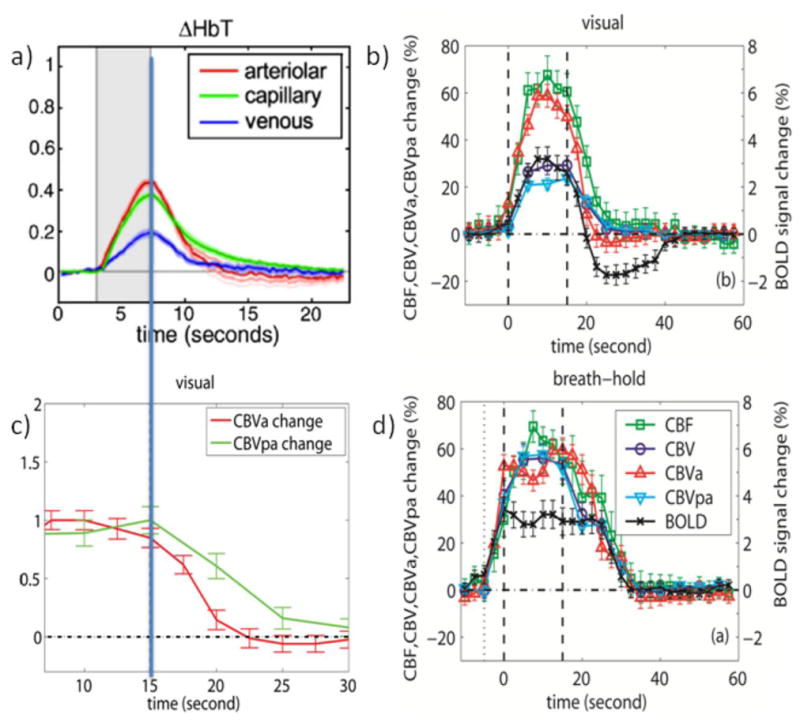
a) Average arteriolar, capillary and venous hemodynamic responses (CBV based on total Hb count) to somatosensory (forepaw) stimulation for 5 rats as obtained from depth-resolved optical imaging and in-vivo two-photon microscopy. Stimulation period in gray. Reprinted from Hillman et al., 2007, Neuroimage 35, 89. b) Average hemodynamic responses (n = 11) for CBF, CBV, CBVa, CBVpa, and GRE-BOLD obtained for visual activation (neuronal stimulation, 15s) in humans. Stimulation periods indicated by vertical dashed lines. c) normalized CBVa and CBVpa responses from (b). d) Average hemodynamic responses (n = 11) during and after brief breath hold (vascular stimulation, 5s exhale + 15s hold). Curves in (b,d) are from significantly activated voxels that overlap for all methods and for both visual and breath hold paradigms and thus of similar tissue/vessel composition. Error bars indicate inter-subject standard deviation. Notice the slow vascular (CBV and CBF) post-stimulus return to baseline for breath hold compared to visual activation as well as the lack of a post-stimulus undershoot following vascular stimulation despite the delayed vascular compliance. Also note the lack of a CBF PSU. Figures b-d were generated from the data used for Figures 1 and 2 of Hua et al., 2011, J. Cereb. Blood Flow Metab. 31, 1599; reprinted with permission.
In view of the above indications that arterial and capillary effects may be important for the BOLD-PSU, the availability of compartmental MRI-CBV methods would be useful. In the tradition that anything can be measured with MRI, such methods have recently been developed for both animal and human studies. Kim et al. developed an arterial CBV approach and used it to confirm that arterial effects dominate CBV changes during somatosensory stimulation in rats (Kim et al., 2007) and visual stimulation in cats (Kim and Kim, 2010, 2011). Their method can be used at high field MRI, where venous blood is invisible due to short T2*. When removing tissue signal (as much as up to 60%) using magnetization transfer (MT) weighting, CBVa can be derived using the knowledge that blood is not affected when irradiating sufficiently off-resonance (Hua et al., 2009; Wolff and Balaban, 1989; Zhou et al., 2005). They did not separate out capillary effects, but mention that part of the early capillaries are included in their CBVa. Consequently, the residual part of the capillaries is included in their venous CBV calculated from the difference of total and arterial CBV. They found a rapidly responding CBVa (potentially with a small post-stimulus undershoot or some BOLD contamination) and a somewhat slower responding CBVtot, both much faster though than the lengthy BOLD undershoot (Kim and Kim, 2010, 2011). When estimating a CBVv curve, it resembled the Mandeville MION curves, but SNR was not sufficient for this curve to comment on the PSU.
Recently, we developed a new inflow VASO (iVASO) approach that is sensitive only to arterial CBV (CBVa) and the arterial transit time (Hua et al., 2011b). Experiments can be performed in a manner to minimize transit time effects, allowing determination of CBVa as well as post-arterial CBV (CBVpa) by combining iVASO and VASO data. When looking at the results for neuronal activation (15 s black and white checker board, Fig. 2b), post-stimulus CBVa returns quickest and CBV, CBVpa and CBF a bit slower. Actually, when normalizing CBVa and CBVpa (Fig. 2c), the curves look very similar to the arterial and capillary data from optical studies (Fig. 2a). Fig. 2d shows CBVa, CBVpa, CBV, CBF and BOLD experiments (Hua et al., 2011a) for vascular CO2 stimulation (15 s breath hold preceded by a 5 s exhaling period). Notice that such short exhale-based breath holds do not cause a PSU, but that longer breath holds may, because of hyperventilation responses. When comparing the hemodynamic response of the vascular activation (Fig. 2d) to that of the neuronal stimulation (Fig. 2b), the CBVa increase is comparable (50–60%) in both tasks. However, while the total relative CBV increase (VASO) is same as CBVa in the vascular task, it is halved in the neuronal paradigm, indicating different vascular mechanisms. This is in line with recent work indicating that pre-capillary and penetrating arterioles but not pericytes mediate functional hyperemia (Fernandez-Klett et al., 2010). On the other hand, the CBF increase is about 60% in both, in agreement with its presumed predominant arteriolar and pre-capillary character. Positive BOLD is also the same for both tasks, namely about 3%. Focusing on the post-stimulus period, it can be seen that recovery is slower in the vascular task where all modalities return simultaneously without a PSU. CBF never goes negative in either paradigm. Most importantly, even though the delay time differs between the two paradigms, CBV, CBVpa and CBF return simultaneously within each paradigm. However, while neuronal stimulation is followed by a prolonged BOLD undershoot that lasts about 15s after these vascular parameters have returned to normal, the vascular stimulation shows no such undershoot. It therefore seems unlikely that vascular effects alone (even CBVpa) will cause a large undershoot. Based on the data in Fig. 2c, we calculated that contributions of delayed CBV return and prolonged increases in oxygen metabolism are about 20% versus 80%, respectively, while CBF effects are negligible even though the CBVa curve may be seen as showing a brief very small PSU (Hua et al., 2011a). The data also rule out an arteriolar compliance effects as the CBVa returns quickly post-stimulus.
A method for measuring “venous” CBV has also been developed (Stefanovic and Pike, 2005), the results of which also show a rapid initial decrease of CBVv directly upon stopping the stimulus, followed by a somewhat slower decay of a remaining small fraction of the initial CBVv response, the latter close to baseline within experimental error (Chen and Pike, 2009). This method is sensitive to deoxygenated blood and, consequently, capillary effects will be included too.
Recently, a combined NIRS-BOLD study of human brain during visual stimulation (Schroeter et al., 2006) was performed, which should provide information about microvascular CBV changes. The NIRS results showed that [Hbtot], typically used as an indicator for CBV in optical studies, did not change post-stimulus within the error of the measurement and it was concluded that most of the BOLD undershoot was due to continuously elevated oxygen metabolism, with a small contribution of CBV remaining ambiguous. Further support for a non-CBV based origin of the PSU comes from recent human experiments showing BOLD-PSU effects in draining larger vessels. An event-related visual stimulation data in humans showed that the ratio of positive BOLD and the BOLD-PSU in V1 was the same in microvascular and macrovascular regions (Zong and Huang, 2011). In another study, the contribution of vascular signals was manipulated using diffusion/flow weighting (Harshbarger and Song, 2008). Again large undershoots were found in vascular regions as well as in deep parenchyma of V1, but, surprisingly, none in higher visual areas. This absence of an undershoot in higher visual areas can be seen as separate evidence against a CBV-based undershoot, because the microvasculature in such regions would be expected to have properties similar to that in V1.
In view of the above optical and MRI results, it seems likely that arteriolar and venular CBV effects rise and return quickly to baseline during and after neuronal stimulation, respectively, while capillary vessels react more slowly, perhaps due to the fact that their regulation is not active (pericyte based) but passive (pressure based) as recently suggested (Fernandez-Klett et al., 2010). Thus a vascular contribution to the PSU most likely would be capillary based. Due to the use of venous terminology that includes both venules and late capillaries as well as arteriolar terminology that includes arterioles and early capillaries, care has to be taken when interpreting the literature.
Spin echo versus gradient echo BOLD undershoots; more compartmentation
Contrary to GRE-BOLD effects, SE-BOLD effects are not sensitive to static dephasing effects (refocused), but are caused by intravascular oxygenation-based T2 changes (Silvennoinen et al., 2003; Thulborn et al., 1982; Wright et al., 1991; J. M. Zhao et al., 2007) and extravascular dynamic averaging (Boxerman et al., 1995; Kiselev and Posse, 1999; Ogawa et al., 1993; Weisskoff et al., 1994). The latter is only significant around the very small microvessels (< ~10 μm). Both diffusion-based SE effects (Jensen et al., 2001) and intravascular SE effects (Silvennoinen et al., 2003; J. M. Zhao et al., 2007) increase quadratically with the field. Due to the big voxels typically used for fMRI in humans, some large vessels are generally included in the measured BOLD effects and for SE effects, these dominate at lower fields (Kennan et al., 1994; Oja et al., 1999). At 1.5T, SE-BOLD is mainly (nearly 100%) intravascular, while this reduces to at most 67% at 3T (Jochimsen et al., 2004). Using a SE sequence without EPI readout (to avoid potential GRE contributions), the Norris group (Poser et al., 2011) recently investigated SE-BOLD effects at 1.5T and 3T and found the same ratio of the positive BOLD effect versus the PSU. In addition, they found no difference in this temporal response positive/negative ratio between SE and GRE effects at 3T. When selecting about 25% of the voxels thought to be mainly large-vessel based, they still detected an undershoot, but about half the size of what was found in the remaining 75%. Similar to the high-resolution animal data (Jin and Kim, 2008b; Yacoub et al., 2006) the existence of an undershoot in the large pial vessels and draining veins indicates a true deoxyhemoglobin change and thus a true OEF change (Eqs. 1,2) independent of CBV effects, which can be explained only by sustained elevation of CMRO2 or reduction in CBF (neglecting Hct and Ya changes). They also argue that if the GRE undershoot would have been caused by CBV effects, a much smaller (around microvessels) or no (around vessels > 10 μm) undershoot would have been visible in the SE effects. This argumentation holds true for extravascular effects, but one has to be careful for intravascular effects, because a delayed recovery of CBV in venules or capillaries could in principle cause a signal undershoot due to the shorter T2 in these partially deoxygenated vessels compared to tissue. This would happen both in SE and GRE data. Since recent optical literature (Berwick et al., 2005; Hillman et al., 2007) is consistently showing negligible venous CBV changes, this would have to be delayed capillary compliance, which would be in line with the data in animals (Kim and Kim, 2010, 2011; Yacoub et al., 2006) and the small post-arterial delay seen by Hua et al. in humans (Fig. 2b). When comparing the visual GRE effects at 3T (Fig. 2) and 1.5T (Fig. 1d) in parenchymal voxels localized by the overlap of CBV and CBF responses, a ratio of positive-BOLD/PSU of about 2/1 is found at 1.5T (TR/TE = 2s/50ms; 5×3.75×3.75 mm3), while this is 3/2 at 3T (TR/TE = 2.5s/45ms; 3×3×3 mm3). Both studies show that better parenchymal localization enhances the BOLD effect (Figs. 1c,d) and reduces this ratio, i.e. increases the PSU contribution. Better localization (3T vs 1.5T) shows increased post-arterial effects (Hua et al., 2011a), but a dominating mismatch between CBV/CBF and BOLD PSU effects remains. Thus, the overriding conclusion remains that a large fraction of the effect is due to prolonged CMRO2 increases.
One note of importance is that the localization of SE effects shifts from predominantly intravascular and macrovascular to predominantly extravascular and microvascular at higher fields (Duong et al., 2003; F. Zhao et al., 2006), providing another opportunity to study region specific fMRI responses.
CBF as a potential contributor to the PSU
There are some suggestions in the literature that post-stimulus CBF undershoots may contribute to the BOLD-PSU (Friston et al., 2000; Hoge et al., 1999; Logothetis et al., 2001; Uludag et al., 2004). This is a logical suggestion based on Eq. 1, showing that a small change in CMRO2, CBF or Hct will have a large effect on venous blood oxygenation. Such BOLD changes can occur without a need for CBV changes, although that would constitute an uncoupling of CBV and CBF unless blood flow is slowed without vascular constriction. Alternatively, it has been hypothesized that a CBF undershoot could be due to vascular constriction due to reduced arteriolar CO2 levels post-stimulation, which would allow CBV-CBF coupling to remain (Yucel et al., 2009).
However, there is a lot of experimental evidence that argues against CBF undershoots. First of all, most groups never measure a significant CBF PSU in humans (Donahue et al., 2009b; Donahue et al., 2009a; Gonzalez-At et al., 2000; Hua et al., 2011a; Kruger et al., 1999; Leontiev et al., 2007; Lu et al., 2004b; Wong et al., 1997; Yang et al., 2000) or animals (Mandeville et al., 1999b; Mandeville et al., 1999a). Secondly, ASL measurements suffer from low SNR (1% signal change ≈ 30–40 ml/100g/min) and even when undershoots are visible, the effect is generally close to the noise level. In addition, older CBF-fMRI hemodynamic response data may have been confounded by the fact that arterial spin labeling based CBF responses are affected by underlying BOLD time courses due to the use of simple control-label subtraction algorithms (Lu et al., 2006), which will cause an apparent undershoot reflecting the BOLD response. Such BOLD interference of course becomes stronger at higher magnetic fields and longer echo times and may cause undershoots in both CBV and CBF experiments. However, more recent studies have started to use surround subtraction and this problem can probably soon be seen as an issue from the past. Thirdly, it is often argued that a post-stimulus local field potential (LFP) undershoot (Logothetis et al., 2001) may cause such a CBF undershoot. However, when analyzing the reported effects in the monkey brain (Logothetis et al., 2001) it is seen that the length of the LFP undershoot (a few seconds) appears to be independent of stimulus duration (4s, 12s and 24s). If the LFP would cause a CBF undershoot, it would not depend on the stimulus duration, which seems contrary to current experimental evidence that the BOLD PSU depends on stimulus length. Of course there is no particular reason to believe that a negative LFP would lead to a flow reduction. It is becoming more established (although often ignored in some of the MRI literature) that the initial CBF increase upon stimulation is not a response to increased CMRO2 demand and traditional neurovascular coupling, but due to neuronal signaling (Attwell and Laughlin, 2001; Attwell et al., 2010; Iadecola and Nedergaard, 2007; Koehler et al., 2009; Lauritzen, 2005). It is unclear whether a negative LFP would induce such signaling. On the other hand, the brief LFP undershoot seems to be of similar length to the CBVa undershoot in Fig. 2a, so a CBF reduction early after the stimulus could contribute.
Recently, in an effort to assess the effects of CBF and CBV, a detailed study of high- and low-intensity visual and sensorimotor stimulation was performed (Chen and Pike, 2009). Venous CBV was measured using the VERVE approach (Stefanovic and Pike, 2005) and CBF using ASL with BOLD-deconvolution. When looking at the group data (Chen and Pike, 2009) for 96 s of activation (Fig. 3), all four stimuli show a rapid post-stimulus reduction in CBVv by 80–90% followed by a somewhat slower decay of the remaining 20–10%, which is close to baseline within standard deviation. The CBF responses either return to baseline (Fig. 3a-c, 2nd row) or show a small brief undershoot (Fig. 3d) sometimes again followed by an overshoot (Fig. 3b,d). The low-intensity stimuli show a clear BOLD PSU without CBF PSU and minimal CBV delays. Importantly, at the time of the BOLD undershoot the CBV appears close to baseline on average, while it goes up at later times when the BOLD PSU has disappeared. This temporal mismatch indicates either a low SNR of the measurement or the fact that CBV delays do not affect the BOLD signal or both. The high intensity sensorimotor data surprisingly show no BOLD PSU, despite the apparent presence of a pronounced delayed CBV return (even though close to error of baseline) and negligible or positive CBF post-stimulus effects. Finally, high-intensity visual stimulation gives the usual BOLD PSU, a CBV delay similar to the other 3 stimuli, and a very brief apparent CBF undershoot. This CBF PSU is about 1/10 of the positive effect during stimulation, easily big enough to explain the BOLD undershoot of ½ of positive BOLD. However, it is followed by an apparent CBF overshoot of about the same size when there is no BOLD PSU or overshoot remaining. Thus our interpretation is that the group data is far from conclusive about any CBV or CBF contributions and more studies will be needed to come to a decisive conclusion whether CBF effects contribute.
Figure 3.
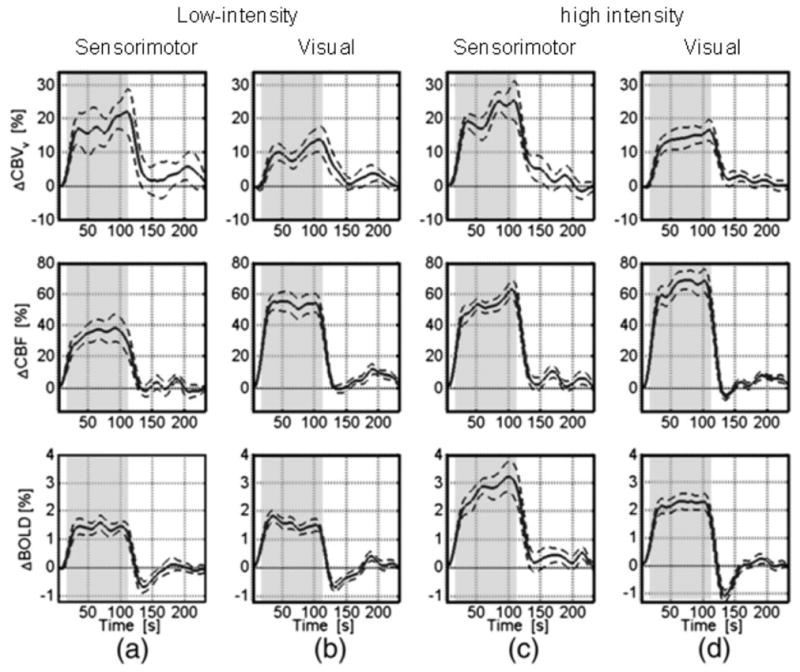
Average time courses for CBVv, CBF and GRE-BOLD obtained during and after 96 s of neuronal activation using (a) low-intensity sensorimotor stimulation (n=11): bilateral tapping at 1.73 Hz; (b) low-intensity visual stimulation (n=13): yellow/blue checker (8 Hz) at 25% contrast; (c) high-intensity (3.46 Hz) sensorimotor stimulation (n=12); (d) high-intensity (8Hz, 100%) visual stimulation (n=14). The dashed lines indicate the standard error, and the stimulation-on period is indicated by the shaded region. Curves are from significantly activated voxels that overlap for all methods, but only the curve with the highest t-value was chosen. In order to enable better visualization of the general transient dynamics, these time courses were smoothed using a low-pass Hanning filter with a 13 s FWHM. Notice the lack of CBF undershoots for sensorimotor and low-intensity visual stimulation as well as fast early CBVv post-stimulus decays followed by very small post-stimulus CBVv variation close to baseline within one standard deviation. Reprinted from Chen and Pike, 2009, Neuroimage. 46, 559, with permission from Elsevier.
More recently, in an interesting study comparing checkerboard visual stimulation with and without flickering at 4 Hz (Sadaghiani et al., 2009), it was found that the PSU disappeared under static presentation. The data also showed a delayed CBF return for static stimulation and an apparent CBF undershoot for flickering, but this was not conclusive with respect to the SNR and perhaps BOLD-convolution. However, stimulation with and without flickering may be an excellent approach to assess the effects of CBF, CBV, CBVa and CBVpa to finally put the PSU debate to rest. With respect to our own data, the lower-SNR 1.5T data in Fig. 1d obtained without BOLD-deconvolution may be seen as suggestive of a CBF undershoot. However, the higher-SNR 3T CBF and CBV data following the visual challenge in Fig. 2c seem to clearly exclude small CBF undershoots. On the other hand, the rapid CBVa decay is followed by what may be a small undershoot. This potential arteriolar constriction may indicate that some CBF effect cannot be totally ruled out, but it may be too small to be measured with the low-SNR ASL technique.
In light of the above considerations, we conclude that evidence regarding a CBF undershoot is still incomplete, mainly due to the fact that ASL is not a very sensitive method in terms of SNR. However, based on the lack of a finding of CBF undershoots by most groups in animals and humans it seems unlikely that CBF undershoots contribute greatly to the BOLD PSU.
Does hematocrit affect BOLD and CBV measurements?
In principle, the Hct (or Hbtot) may affect both intravascular (Eqs. 1,2) and extravascular (Eq. [3]) BOLD signal changes (Eqs. 1–3). MRI signal changes based on paramagnetic contrast agents are also sensitive to Hct (Eq. [4]). Thus, a Hct change could cause several of the effects seen in BOLD studies or the MION-CBV experiments. For instance, a post-stimulus reduction in Hct would show a BOLD undershoot and the MION-based CBV signal would show a prolonged return. When Hct changes, the blood T1 will change and the VASO signal may get affected. However, we currently don’t have proper techniques to assess whether Hct changes occur during stimulation or post-stimulation. As a result, both MRI and optical studies of CBV generally assume a constant Hct during neuronal activation, as do BOLD-MRI studies. It is currently unclear whether Hct contributes substantially to either the positive BOLD response or the PSU.
Have sustained post-stimulus CMRO2 increases actually been measured?
Even though the arguments ruling out major BOLD-PSU contributions from vascular compliance and CBF are strong, the interpretation of sustained CMRO2 increases has a weakness in that CMRO2 has not been measured directly with MRI or optical approaches. After being suggested as a possible mechanism in the nineties based purely on BOLD data (Frahm et al., 1996), the earliest experimental MRI evidence (Fig. 1d) was based on human BOLD, CBF, and CBV measurements (Lu et al., 2004b). The absence of significant CBV and CBF changes during the PSU was interpreted as evidence for sustained CMRO2 increases as based on Eq. 1 (assuming negligible changes in Hct). CMRO2 and OEF were then determined using more complicated multi-compartment modeling of vasculature (arterioles/venules) and tissue, but the overall conclusion was based on simple physiological considerations. Using these same scientific arguments, this conclusion was later substantiated in humans (Dechent et al., 2011; Donahue et al., 2009a; Frahm et al., 2008; Hua et al., 2011a; Poser et al., 2011) and in animals (Jin and Kim, 2008b; Yacoub et al., 2006). Especially the high-resolution animal experiments where BOLD-PSUs were localized in surface pial vessel regions are important, because no delayed CBV effects were measured there during the BOLD-PSU. This rules out venous vascular compliance as a potential origin for such regions and the deoxygenation measured during the BOLD-PSU can only be due to an actual oxygenation effect inside the vessel. The original explanation of delays in venous vascular compliance was that venous blood oxygenation had returned to normal but an undershoot is seen due to increased CBV. However, even if blood of normal venous oxygenation is delayed inside deep cortex, no undershoot will be seen in the surface vessel regions if there is no CBV change there. Thus, such a surface-vessel undershoot in absence of surface vessel CBV change has to due to a true OEF contribution and thus either a CBF reduction or CMRO2 increase (assuming constant Hct and Ya) that occurred in the contributing parenchyma as reflected in the exact equation 1 above. Interestingly, if an oxygenation change has occurred in the deep cortex and the venous vessels in there have delayed recovery, the undershoot may appear to be longer in the surface vessels than in the deep cortex. An alternative possibility is that OEF changes from deeper areas that are not involved in the activation can contribute, but this seems unlikely, because the cortex draining into the surface was selected for the deep-cortex hemodynamic data in these studies. The current evidence could be improved if intravascular BOLD-PSU data could be obtained from draining veins, because such data would certainly rule out CBV contributions. Such experiments should be possible at high magnetic fields.
In an effort to separate out the vascular and most of the CBF contributions from BOLD signal changes, two groups have performed experiments under conditions of close to maximal vasodilatation, either using hypotension (Nagaoka et al., 2006) or hypercapnia with 6% CO2 (Zappe et al., 2008). In both studies, the positive BOLD response disappeared and a negative, CMRO2-dominated response remained, the length of which was similar to the positive BOLD response. Unfortunately, neither of these papers studied the BOLD-PSU in detail. The Zappe study showed a small undershoot during normocapnia that appears to coincide with part of the negative BOLD effect during hypercapnia, but this was not mentioned in the paper and the limited dataset does not provide unequivocal proof. However, studies such as these but then focused on regions with a pronounced undershoot under normocapnia would be important to perform.
The most direct non-MRI evidence supporting continued increases in CMRO2 comes from data showing prolonged post-stimulus pO2 reductions in animals (Ances et al., 2001) and perfused tissue (Brockhaus et al., 1993), the time scales of which are of the same order as BOLD-PSUs for similar lengths of stimulation.
Back to square one?
Recently, several studies were published that seem to disprove sustained CMRO2 as a cause of the BOLD undershoot or show more proof for a venous vascular compliance model.
First, in 2010, some experiments were published employing LDF and optical spectroscopy to study the effect of slightly increased intracranial pressure (ICP) on neurovascular coupling during somatosensory stimulation in rats (Fuchtemeier et al., 2010). Despite an increase in baseline CBF of 92% at an ICP of 28 mmHg, these studies still showed additional increases in CBF (~12%) and CBV (~5%) during stimulus as well as an increase in deoxyhemoglobin (~4%) concentration, corresponding to a large CMRO2 increase during activation. However, deoxyhemoglobin, CBF or CBV were found to return to baseline post-stimulus. Under the assumption that continued post-stimulus increases in CMRO2 should also occur under these conditions if they are needed to restore the system, the absence of a dHb overshoot was concluded to reflect that continued increases in CMRO2 cannot contribute to the BOLD post-stimulus undershoot and that it has to be a vascular phenomenon. However, this study found a large increase in CBF (92%) without an increase in baseline CBV, which seems inconsistent with known physiology. When using infusions of CSF to moderately increase ICP, downstream cortical bridging veins entering the sagittal sinus are compressed. This should cause some distension of downstream large veins and possibly venules in the field of view, leading to increases in baseline CBV without decreasing baseline CBF. The finding of increased CBF without increased arterial blood volume under conditions of increased downstream pressure is contradictory to expectation. Also, if venous blood volume is not increased by increased ICP, then it seems unlikely that a change in the post-stimulus deoxyhemoglobin is attributable to a change in vascular compliance.
Recently, several papers appeared indicating that the arterial/arteriolar response to stimulation is fast and that the venous response is slower and may only become visible after longer stimulation (Berwick et al., 2008; Drew et al., 2011; Hirano et al., 2011; Kim and Kim, 2011). As the BOLD-PSU has been reported to be a stimulus-duration dependent phenomenon, this could be one of the likely causes. Also, the contrast-agent based CBV measurements are dominated by arterial CBV effects which return to baseline rapidly after stimulation and may overshadow smaller CBVv contributions. Thus the iVASO and agent-based total CBV measurements may hide the delayed venous effects. When separating out arterial effects, such CBVv contributions should become visible. This was seen in Fig. 2b (CBVpa) for the 15 s stimulation and may be more pronounced for longer ones. Also, the arterial CBV undershoot seems to be short. As such, the relative contributions of sustained CMRO2, delayed vascular compliance, and even CBF undershoot may vary with stimulus length and type and more studies are still needed to acquire a detailed understanding of the BOLD_PSU phenomenon.
Conclusions
The BOLD-PSU has been a topic of heated discussion for most of the existence of fMRI. During these two decades more data has become available due to the continued expansion of available MR technology, which now includes noninvasive measurement of arteriolar, venous (post-arteriolar) and total CBV changes. These data, together with results from optical studies provide strong arguments to refute a BOLD-PSU explanation in terms of a dominant contribution of delayed venous compliance to the PSU. First, high-resolution animal studies show the clear presence of a BOLD-PSU in selected large-vessel areas of parenchyma in absence of measurable CBV changes, which can in principle only be explained by a pure deoxyhemoglobin increase and thus OEF effect (Eq. 1). This phenomenon indicates that an uncoupling of OEF and CBV has occurred. Second, there is no convincing evidence of a CBF-PSU in most reported human and animal studies, and even in the few studies that appear to detect such an effect, the CBF effects are generally close to within the standard deviation of the measurement. Thus, under the assumption of constant Hct and in the absence of measurable CBV and CBF effects, the OEF effects in large draining vessels appear to be due mainly to sustained elevation of CMRO2. Some small contributions of delayed post-arteriolar (capillary or venular/venous) compliance are likely, while small CBF undershoots have not yet been ruled out due to the typically low SNR of ASL experiment. Such a large contribution of CMRO2 under conditions of normalized CBF and CBV refutes the oxygen-limitation basis of the balloon model.
Some caveats have to be made, however. First, recent studies show a fast and brief arteriolar CBV decline post-stimulus, which could be related to the short LFP undershoot measured in monkeys, and it is premature to rule out a partial CBF contribution completely. Improvement in the SNR of ASL technology is needed to provide more clarity. Also, if such an arterial CBV undershoot occurs, it may possibly cover up the venous delayed return when measuring total CBV with either a contrast agent or VASO. Second, a main factor complicating the interpretation of BOLD-PSU data is that the contribution of arteriolar, capillary and venular compartments to CBV changes vary with the length of stimulation, while the BOLD and total CBV approaches measure the combined effect of all compartments. Optical data show that vascular changes during the onset of brain activation occur predominantly in arterioles and capillaries, while recent MRI and optical data show a delayed venous CBV response that increases with stimulation duration. It therefore seems likely that the contributions of the three possible mechanisms (sustained CMRO2 increase, CBF-PSU and CBVpa delayed compliance) may vary with stimulation length and type. No doubt there will be more discussions and data forthcoming before the picture will be totally clear.
Acknowledgments
Grant support from NIH-NCRR P41-RR15241, NIH-NIMH R01-MH084021.
We are grateful to Dr. Ray Koehler (Johns Hopkins University, Department of Anesthesiology and Critical Care) for insightful comments with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ances BM, Buerk DG, Greenberg JH, Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci Lett. 2001;306:106–10. doi: 10.1016/s0304-3940(01)01868-7. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–5. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Liu TT. An arteriolar compliance model of the cerebral blood flow response to neural stimulus. Neuroimage. 2005;25:1100–11. doi: 10.1016/j.neuroimage.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JE. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. Eur J Neurosci. 2005;22:1655–66. doi: 10.1111/j.1460-9568.2005.04347.x. [DOI] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Martin C, Kennerley AJ, Redgrave P, Mayhew JE. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J Neurophysiol. 2008;99:787–98. doi: 10.1152/jn.00658.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med. 1995;34:555–66. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J Physiol. 1993;462:421–45. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–64. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–33. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. Origins of the BOLD post-stimulus undershoot. Neuroimage. 2009;46:559–68. doi: 10.1016/j.neuroimage.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Dechent P, Schutze G, Helms G, Merboldt KD, Frahm J. Basal cerebral blood volume during the poststimulation undershoot in BOLD MRI of the human brain. J Cereb Blood Flow Metab. 2011;31:82–9. doi: 10.1038/jcbfm.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Stevens RD, de Boorder M, Pekar JJ, Hendrikse J, van Zijl PC. Hemodynamic changes after visual stimulation and breath holding provide evidence for an uncoupling of cerebral blood flow and volume from oxygen metabolism. J Cereb Blood Flow Metab. 2009a;29:176–85. doi: 10.1038/jcbfm.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Blicher JU, Ostergaard L, Feinberg DA, MacIntosh BJ, Miller KL, Gunther M, Jezzard P. Cerebral blood flow, blood volume, and oxygen metabolism dynamics in human visual and motor cortex as measured by whole-brain multimodal magnetic resonance imaging. J Cereb Blood Flow Metab. 2009b;29:1856–66. doi: 10.1038/jcbfm.2009.107. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc Natl Acad Sci U S A. 2011;108:8473–8. doi: 10.1073/pnas.1100428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Kim SG. Microvascular BOLD contribution at 4 and 7 T in the human brain: gradient-echo and spin-echo fMRI with suppression of blood effects. Magn Reson Med. 2003;49:1019–27. doi: 10.1002/mrm.10472. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–5. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Kruger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–8. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Frahm J, Baudewig J, Kallenberg K, Kastrup A, Merboldt KD, Dechent P. The post-stimulation undershoot in BOLD fMRI of human brain is not caused by elevated cerebral blood volume. Neuroimage. 2008;40:473–81. doi: 10.1016/j.neuroimage.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage. 2000;12:466–77. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Fuchtemeier M, Leithner C, Offenhauser N, Foddis M, Kohl-Bareis M, Dirnagl U, Lindauer U, Royl G. Elevating intracranial pressure reverses the decrease in deoxygenated hemoglobin and abolishes the post-stimulus overshoot upon somatosensory activation in rats. Neuroimage. 2010;52:445–54. doi: 10.1016/j.neuroimage.2010.04.237. [DOI] [PubMed] [Google Scholar]
- Gonzalez-At JB, Alsop DC, Detre JA. Cerebral perfusion and arterial transit time changes during task activation determined with continuous arterial spin labeling. Magn Reson Med. 2000;43:739–46. doi: 10.1002/(sici)1522-2594(200005)43:5<739::aid-mrm17>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Harshbarger TB, Song AW. Differentiating sensitivity of post-stimulus undershoot under diffusion weighting: implication of vascular and neuronal hierarchy. PLoS One. 2008;3:e2914. doi: 10.1371/journal.pone.0002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Stefanovic B, Silva AC. Spatiotemporal evolution of the functional magnetic resonance imaging response to ultrashort stimuli. J Neurosci. 2011;31:1440–7. doi: 10.1523/JNEUROSCI.3986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Stimulus-dependent BOLD and perfusion dynamics in human V1. Neuroimage. 1999;9:573–85. doi: 10.1006/nimg.1999.0443. [DOI] [PubMed] [Google Scholar]
- Hua J, Stevens RD, Huang AJ, Pekar JJ, van Zijl PC. Physiological origin for the BOLD poststimulus undershoot in human brain: vascular compliance versus oxygen metabolism. J Cereb Blood Flow Metab. 2011a;31:1599–611. doi: 10.1038/jcbfm.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Qin Q, Donahue MJ, Zhou J, Pekar JJ, van Zijl PC. Inflow-based vascular-space-occupancy (iVASO) MRI. Magn Reson Med. 2011b;66:40–56. doi: 10.1002/mrm.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Donahue MJ, Zhao JM, Grgac K, Huang AJ, Zhou J, van Zijl PC. Magnetization transfer enhanced vascular-space-occupancy (MT-VASO) functional MRI. Magn Reson Med. 2009;61:944–51. doi: 10.1002/mrm.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Chandra R, Yu H. Quantitative model for the interecho time dependence of the CPMG relaxation rate in iron-rich gray matter. Magn Reson Med. 2001;46:159–65. doi: 10.1002/mrm.1171. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG. Improved cortical-layer specificity of vascular space occupancy fMRI with slab inversion relative to spin-echo BOLD at 9.4 T. Neuroimage. 2008a;40:59–67. doi: 10.1016/j.neuroimage.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008b;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochimsen TH, Norris DG, Mildner T, Moller HE. Quantifying the intra- and extravascular contributions to spin-echo fMRI at 3 T. Magn Reson Med. 2004;52:724–32. doi: 10.1002/mrm.20221. [DOI] [PubMed] [Google Scholar]
- Jones RA. Origin of the signal undershoot in BOLD studies of the visual cortex. NMR Biomed. 1999;12:299–308. doi: 10.1002/(sici)1099-1492(199908)12:5<299::aid-nbm566>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Jones RA, Schirmer T, Lipinski B, Elbel GK, Auer DP. Signal undershoots following visual stimulation: a comparison of gradient and spin-echo BOLD sequences. Magn Reson Med. 1998;40:112–8. doi: 10.1002/mrm.1910400116. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Eleff SM, Ulatowski JA, Kraut M, Soher B, van Zijl PC. Visual activation in alpha-chloralose-anaesthetized cats does not cause lactate accumulation in the visual cortex as detected by [1H]NMR difference spectroscopy. Eur J Neurosci. 1997;9:654–61. doi: 10.1111/j.1460-9568.1997.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med. 1994;31:9–21. doi: 10.1002/mrm.1910310103. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40:840–6. doi: 10.1002/mrm.1910400609. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SG. Cortical layer-dependent arterial blood volume changes: improved spatial specificity relative to BOLD fMRI. Neuroimage. 2010;49:1340–9. doi: 10.1016/j.neuroimage.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Kim SG. Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow Metab. 2011;31:1211–22. doi: 10.1038/jcbfm.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Hendrich KS, Masamoto K, Kim SG. Arterial versus total blood volume changes during neural activity-induced cerebral blood flow change: implication for BOLD fMRI. J Cereb Blood Flow Metab. 2007;27:1235–47. doi: 10.1038/sj.jcbfm.9600429. [DOI] [PubMed] [Google Scholar]
- Kiselev VG, Posse S. Analytical model of susceptibility-induced MR signal dephasing: effect of diffusion in a microvascular network. Magn Reson Med. 1999;41:499–509. doi: 10.1002/(sici)1522-2594(199903)41:3<499::aid-mrm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Koch RA, Barish ME. Perturbation of intracellular calcium and hydrogen ion regulation in cultured mouse hippocampal neurons by reduction of the sodium ion concentration gradient. J Neurosci. 1994;14:2585–93. doi: 10.1523/JNEUROSCI.14-05-02585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kruger G, Kleinschmidt A, Frahm J. Dynamic MRI sensitized to cerebral blood oxygenation and flow during sustained activation of human visual cortex. Magn Reson Med. 1996;35:797–800. doi: 10.1002/mrm.1910350602. [DOI] [PubMed] [Google Scholar]
- Kruger G, Kastrup A, Takahashi A, Glover GH. Simultaneous monitoring of dynamic changes in cerebral blood flow and oxygenation during sustained activation of the human visual cortex. Neuroreport. 1999;10:2939–43. doi: 10.1097/00001756-199909290-00012. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Hopkins AL, Haacke EM, Li D, Wasserman BA, Buckley P, Friedman L, Meltzer H, Hedera P, Friedland R. Identification of vascular structures as a major source of signal contrast in high resolution 2D and 3D functional activation imaging of the motor cortex at 1.5T: preliminary results. Magn Reson Med. 1993;30:387–92. doi: 10.1002/mrm.1910300318. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB, Mandeville JB. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–94. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. Neuroimage. 2007;36:1110–22. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lu H, van Zijl PC. Experimental measurement of extravascular parenchymal BOLD effects and tissue oxygen extraction fractions using multi-echo VASO fMRI at 1.5 and 3.0 T. Magn Reson Med. 2005;53:808–16. doi: 10.1002/mrm.20379. [DOI] [PubMed] [Google Scholar]
- Lu H, van Zijl PC. A review of the development of Vascular-Space-Occupancy (VASO) fMRI. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2012.01.013. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Donahue MJ, van Zijl PC. Detrimental effects of BOLD signal in arterial spin labeling fMRI at high field strength. Magn Reson Med. 2006;56:546–52. doi: 10.1002/mrm.20976. [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263–74. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004a;52:679–82. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PC. Sustained poststimulus elevation in cerebral oxygen utilization after vascular recovery. J Cereb Blood Flow Metab. 2004b;24:764–70. doi: 10.1097/01.WCB.0000124322.60992.5C. [DOI] [PubMed] [Google Scholar]
- Lu H, Scholl CA, Zuo Y, Stein EA, Yang Y. Quantifying the blood oxygenation level dependent effect in cerebral blood volume-weighted functional MRI at 9.4T. Magn Reson Med. 2007;58:616–21. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999a;42:944–51. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–24. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab. 1999b;19:679–89. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Zhao F, Wang P, Harel N, Kennan RP, Ogawa S, Kim SG. Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition. J Cereb Blood Flow Metab. 2006;26:1043–51. doi: 10.1038/sj.jcbfm.9600251. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–12. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja JM, Gillen J, Kauppinen RA, Kraut M, van Zijl PC. Venous blood effects in spin-echo fMRI of human brain. Magn Reson Med. 1999;42:617–26. doi: 10.1002/(sici)1522-2594(199910)42:4<617::aid-mrm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Poser BA, van Mierlo E, Norris DG. Exploring the post-stimulus undershoot with spin-echo fMRI: implications for models of neurovascular response. Hum Brain Mapp. 2011;32:141–53. doi: 10.1002/hbm.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Ugurbil K, Uludag K. Neural activity-induced modulation of BOLD poststimulus undershoot independent of the positive signal. Magn Reson Imaging. 2009;27:1030–8. doi: 10.1016/j.mri.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Kupka T, Mildner T, Uludag K, von Cramon DY. Investigating the post-stimulus undershoot of the BOLD signal--a simultaneous fMRI and fNIRS study. Neuroimage. 2006;30:349–58. doi: 10.1016/j.neuroimage.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP, Duyn JH. Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex. Magn Reson Med. 2007;57:1110–8. doi: 10.1002/mrm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PC. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med. 2003;49:47–60. doi: 10.1002/mrm.10355. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Pike GB. Venous refocusing for volume estimation: VERVE functional magnetic resonance imaging. Magn Reson Med. 2005;53:339–47. doi: 10.1002/mrm.20352. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, Silva AC. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab. 2008;28:961–72. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265–70. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Tuunanen PI, Vidyasagar R, Kauppinen RA. Effects of mild hypoxic hypoxia on poststimulus undershoot of blood-oxygenation-level-dependent fMRI signal in the human visual cortex. Magn Reson Imaging. 2006;24:993–9. doi: 10.1016/j.mri.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Uludag K, Muller-Bierl B, Ugurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage. 2009;48:150–65. doi: 10.1016/j.neuroimage.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage. 2004;23:148–55. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4:159–67. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med. 1994;31:601–10. doi: 10.1002/mrm.1910310605. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–44. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–49. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi Award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imaging. 1991;1:275–83. doi: 10.1002/jmri.1880010303. [DOI] [PubMed] [Google Scholar]
- Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32:749–63. doi: 10.1002/mrm.1910320610. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Ugurbil K, Harel N. The spatial dependence of the poststimulus undershoot as revealed by high-resolution BOLD- and CBV-weighted fMRI. J Cereb Blood Flow Metab. 2006;26:634–44. doi: 10.1038/sj.jcbfm.9600239. [DOI] [PubMed] [Google Scholar]
- Yang Y, Engelien W, Pan H, Xu S, Silbersweig DA, Stern E. A CBF-based event-related brain activation paradigm: characterization of impulse-response function and comparison to BOLD. Neuroimage. 2000;12:287–97. doi: 10.1006/nimg.2000.0625. [DOI] [PubMed] [Google Scholar]
- Yucel MA, Devor A, Akin A, Boas DA. The Possible Role of CO(2) in Producing A Post-Stimulus CBF and BOLD Undershoot. Front Neuroenergetics. 2009;1:7. doi: 10.3389/neuro.14.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe AC, Uludag K, Logothetis NK. Direct measurement of oxygen extraction with fMRI using 6% CO2 inhalation. Magn Reson Imaging. 2008;26:961–7. doi: 10.1016/j.mri.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, Kim SG. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage. 2006;30:1149–60. doi: 10.1016/j.neuroimage.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Zhao JM, Clingman CS, Narvainen MJ, Kauppinen RA, van Zijl PC. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med. 2007;58:592–7. doi: 10.1002/mrm.21342. [DOI] [PubMed] [Google Scholar]
- Zhou J, Payen JF, van Zijl PC. The interaction between magnetization transfer and blood-oxygen-level-dependent effects. Magn Reson Med. 2005;53:356–66. doi: 10.1002/mrm.20348. [DOI] [PubMed] [Google Scholar]
- Zong X, Huang J. Linear coupling of undershoot with BOLD response in ER-fMRI and nonlinear BOLD response in rapid-presentation ER-fMRI. Neuroimage. 2011;57:391–402. doi: 10.1016/j.neuroimage.2011.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]


