Figure 7. Proposed model for Lst2 function in EGFR endocytosis.
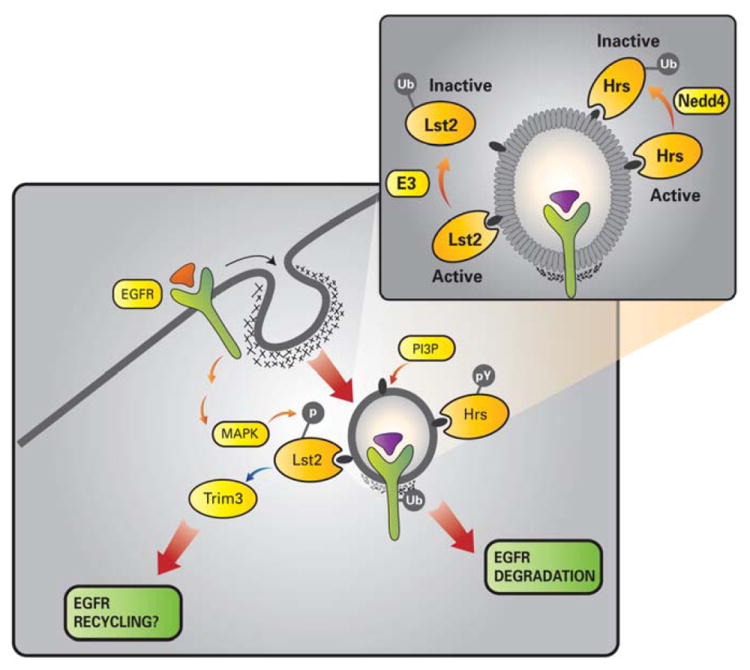
The scheme depicts localization of the newly described Lst2 protein to PI3P-enriched early endosomes, by virtue of its FYVE domain. Lst2 undergoes monoubiquitinylation at a conserved lysine residue (lysine 87), which results in impairment of its FYVE domain, and sequestration from endosomes (inset). Accordingly, we envisage an ubiquitinylation/deubiquitinylation cycle that allows intermittent recruitment of Lst2 to sorting endosomes during EGFR trafficking. In comparison, the extensively characterized FYVE domain protein, Hrs, remains membrane bound despite its ubiquitinylation by Nedd4 family E3 ubiquitin ligases. Note that EGF-induced and MAPK-mediated serine/threonine phosphorylation of Lst2 contrasts with tyrosine phosphorylation of Hrs. We find that Lst2 associates with the RBCC protein, Trim3, which in turn couples to the CART cargo recycling complex, comprising α-actinin-4 and myosin V.
