Abstract
Background:
Little is known about the relation between preoperative glycemic state and neurosurgical outcomes. Improved understanding of this relationship may identify patients at increased risk of complicated recovery and guide postoperative treatment strategies.
Methods:
Data were collected about 918 consecutive craniotomy or spine-related neurosurgical cases at the University of Michigan Hospitals. Univariate statistics, bivariate chi-square tests, and analysis of variance were used to assess relations between preoperative blood glucose, demographics, medical comorbidities, systemic glucocorticoid use, and postoperative complication risk and postoperative hospital and intensive care unit (ICU) stay. We fit a multivariable logistic regression model of 30-day complication risk by preoperative blood glucose adjusted for potential confounders, and used analysis of covariance to assess the relation between preoperative blood glucose and hospital, as well as ICU stay, adjusted for potential confounders.
Results:
Among all patients, 56.1% had peri-operative blood glucose levels below 100 mg/dl. 20.7% had levels from 100 to 120 mg/dl, 16.3% had levels from 121 to 160 mg/dl, and 6.9% had levels greater than 160 mg/dl. In multivariable regression models, blood glucose greater than 120 mg/dl was associated with increased risk of postoperative complications at all levels. Analysis of covariance showed that preoperative blood glucose above 120 mg/dl was associated with both increased length of ICU stay and length of hospital stay.
Conclusions:
Our findings suggest that even mild preoperative hyperglycemia is a predictor of postoperative complication risk, and prolonged hospital and ICU stay following neurosurgical intervention. Tight glycemic control may be in order when attempting to reduce risk of complications and limit postoperative recovery time.
Keywords: Complications, diabetes mellitus, glucose, hospital stay, hyperglycemia, neurosurgery
INTRODUCTION
Diabetes mellitus (DM) is a growing epidemic in the United States and other high-income countries.[34] Aging populations and increasing obesity rates are expected to result in a doubling of the number of people with diabetes worldwide by 2030.[34] Moreover, hyperglycemia in the absence of formal DM diagnoses is also on the rise,[30] with most individuals with hyperglycemia eventually moving on to develop diabetes.[32] The deleterious effects of hyperglycemia on human health are well known, as hyperglycemia is predictive of both microvascular and macrovascular diseases.[28]
The increasing prevalence and complexity of neurosurgical intervention have necessitated a more precise understanding of the determinants of postoperative complications. Understanding the risk factors for postoperative complications may assist clinicians in taking preventative measures against complication, and may allow for more accurate surgical risk assessments. Given the increasing risk for hyperglycemia and DM in the general population, understanding the influence of blood glucose on postoperative outcome following neurosurgery is of increasing importance.
The literature about this relation lacks consensus. Several well-designed studies in the spinal literature have demonstrated a deleterious effect of DM on wound healing.[6,19,29,11] However, the effect of DM on postoperative complication risk is contested in craniotomy studies.[25,26] Moreover, a variety of studies have suggested that the level of diabetic control, rather than diabetic status alone, may be an important contributor to postoperative complication risk.[16] In trauma patients, preoperative blood glucose has been shown to predict both morbidity and mortality following surgery.[5] While the measurement of hemoglobin A1c, the metric most often used in the extant literature, provides an excellent reference for long-term control of blood sugar, acute preoperative hyperglycemia may also be an important predictor of post-surgical outcomes.
MATERIALS AND METHODS
Defining exposures and outcomes of interest
We collected retrospective data about 918 consecutive craniotomy or spine-related neurosurgical cases which had the surgery conducted at the University of Michigan Hospitals from April 10, 2006 through May 4, 2009. The following factors were causes for patient removal from this study: patients under 18 years of age, patients lacking 30-day follow-up, patients with incomplete medical records, patients undergoing only ventriculostomy, any head or neck cases which did not include craniotomy or spinal procedures, and any neuroendovascular procedures. Out of an original cohort of 1331 adult patients, 918 were found suitable for inclusion in our final analysis.
We collected data about preoperative blood glucose, patient demographics, comorbidities, postoperative complications, length of ICU stay, and length of hospital stay from neurosurgery clinical notes. Complete data points were available for all variables for all patients found suitable for inclusion. Data were collected by trained medical students using full clinical registers in the electronic medical record system of the University of Michigan. Demographic and comorbidity data were taken from standard questions asked in every preoperative anesthesia and surgical notes, as well as from previous clinical records. Intraoperative data were taken from anesthesia records and surgical reports. Postoperative complication data were taken from daily progress notes as well as from discharge notes. Blood glucose was obtained as a fasting value from standard preoperative labs. The most recent preoperative values (relative to time of surgery) were analyzed for traumatic and emergent cases. Blood glucose was analyzed as a categorical variable: <100 mg/dl, 100–120 mg/dl, 121–160 mg/dl, and >160 mg/dl. Demographic data compiled included gender, age (analyzed as a categorical variable: <50 years, 50–70 years, and 71 years or older), and Body Mass Index (BMI, analyzed as a categorical variable: <30 kg/m2, 30–40 kg/m2, and >40 kg/m2). Comorbidity data collected included previous diagnosis with DM (analyzed as a binary variable: yes or no). Medical covariates included use of insulin (analyzed as a binary variable: yes or no), use of other medications for DM (analyzed as a binary variable: yes or no), and chronic glucocorticoid use (analyzed as a binary variable: yes or no). Other clinical covariates considered included emergent cases (defined as those requiring immediate neurosurgical interventions without regard to the underlying disorder, analyzed as a binary variable: yes or no), trauma (analyzed as a binary variable: yes or no), and case type (analyzed as a binary variable: spinal or craniotomy).
Complications were defined as any of the following within 30 days following operation: death, coma >24 h, acute renal failure, postoperative bleeding requiring ICU stay, reoperation, >4 units RBCs within 72 h, unplanned intubation, ventilation lasting greater than 48 h, pneumonia, cardiac arrest, myocardial infarction, pulmonary embolus, deep infection, sepsis, systemic inflammatory response syndrome (SIRS), pseudomeningocele, deep vein thrombosis, seizure, and cerebrovascular accident (complications were analyzed as one binary variable denoting the presence or absence of any complication). These complications were further subdivided into the following categories: neurological, cardiovascular, infectious, reoperation, and other for subgroup analysis. Both length of ICU stay and total length of hospital stay were recorded in days for each patient. These variables were analyzed as continuous dependent variables.
This study was reviewed by the Medical Science Institutional Review Board of the University of Michigan.
Analysis
First, we calculated univariate statistics to describe our sample. Second, we used bivariate chi-square tests to identify significant associations between the covariates under study and postoperative complication rates, as well as analysis of variance (ANOVA) to determine significant associations between covariates and continuous outcomes, length of hospital stay, and length of ICU stay. Third, we fit a multivariable logistic regression model of 30-day complication risk by preoperative blood glucose adjusted for potential confounders. Fourth, we performed analysis of covariance (ANCOVA) to determine significant associations between covariates of interest and continuous outcomes, adjusting for all potential confounders, as well as the occurrence of a postoperative complication.
Only associations with P <0.05 were taken as significant. SAS 9.2 (SAS Institute, Cary, NC, USA) was used to carry out all statistical analyses.
RESULTS
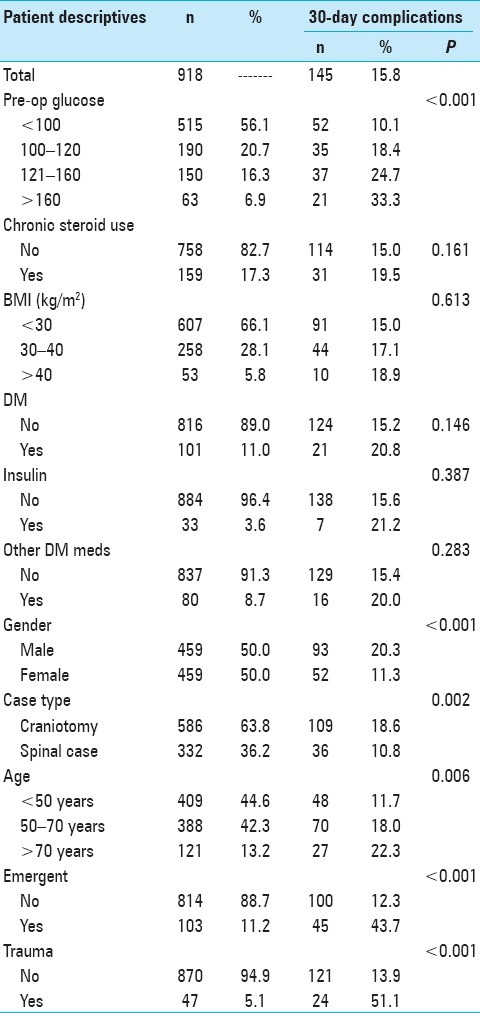
Table 1 shows the demographic characteristics and bivariate chi-square tests between all covariates and 30-day complication risk. Of the 918 patients in our study, 145 (15.8%) experienced a major complication within 30 postoperative days. Preoperative blood glucose, gender, case type, increased age, emergent cases, and traumatic cases were all associated with 30-day risk of postoperative complications. All preoperative blood glucose levels >100 mg/dl were associated with higher complication risk than preoperative blood glucose levels <100 mg/dl. There appeared to be a dose-response relationship between blood glucose and complication risk (33.3% complication rate for patients with preoperative blood glucose >160 mg/dl, 24.7% complication rate for patients with preoperative blood glucose 121–160 mg/dl, 18.4% complication rate for patients with preoperative blood glucose 100–120 mg/dl, and 10.1% complication rate for patients with preoperative blood glucose <100 mg/dl). Male gender was associated with higher complication risk than female gender (20.3% vs. 11.3%, P < 0.001), craniotomy cases were associated with higher complication risk than spinal cases (18.6% vs. 10.8%, P = 0.002), and ages 50–70 years and >70 years were both associated with higher complication risk than age <50 years (P = 0.006). Emergent cases had higher complication rates than scheduled cases (43.7% complication rate vs. 12.3%, P < 0.001), while traumatic cases had higher complication rates than non-traumatic cases (51.1% vs. 13.9%, P < 0.001). Chronic steroid use, BMI, DM, use of insulin, and use of other DM medications were not associated with 30-day postoperative complication risk.
Table 1.
Descriptive statistics and bivariate Chi-square tests between explanatory covariates and post-surgical complication risk among 918 patients undergoing neurosurgical intervention at the University of Michigan Hospitals
We further subdivided postoperative complications into neurological, cardiovascular, infectious, reoperation, and other [Figure 1]. In bivariate chi-square analysis, glucose 120–160 mg/dl predisposed to neurological (P = 0.028), cardiovascular (P = 0.042), and infectious (P = 0.026) complications relative to glucose <100 mg/dl. Glucose >160 mg/dl predisposed to neurological (P = 0.002), cardiovascular (P = 0.046), infectious (P = 0.022), and other (P = 0.046) complications relative to glucose <100 mg/dl.
Figure 1.
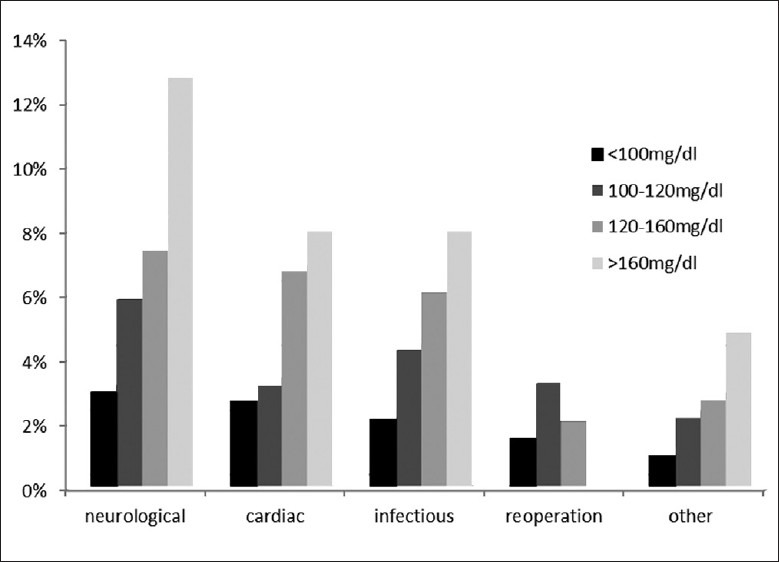
30-day postoperative complication rates as a function of preoperative blood glycemia values among 918 neurosurgical patients at the University of Michigan Hospitals
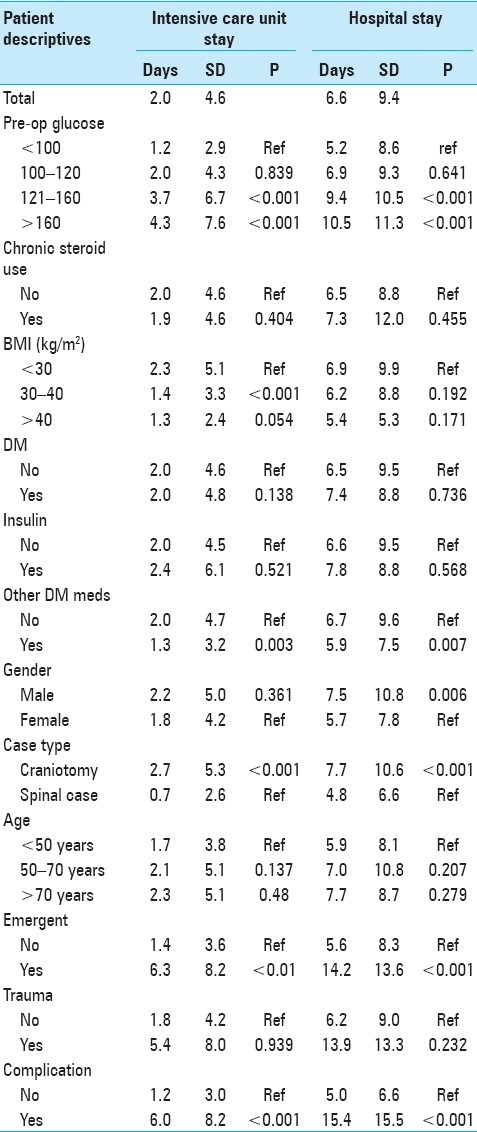
Table 2 shows mean post-surgical stay in hospital and ICU, as well as ANOVA between covariates of interest and post-surgical hospital and ICU stay among patients in our sample. For all patients, the mean length of stay in the ICU was 2.0 days [standard deviation (SD) 4.6], while the mean length of stay in the hospital was 6.6 days (SD 9.4). Increased preoperative blood glucose was associated with increased length of ICU stay in a dose–response fashion (average of 4.3 days for patients with preoperative blood glucose >160 mg/dl, 3.7 days for patients with preoperative blood glucose 121–160 mg/dl, 2.0 days for patients with preoperative blood glucose 100–120 mg/dl, 1.2 days for patients with preoperative blood glucose <100 mg/dl, P < 0.001). Preoperative blood glucose was also associated with increased length of hospital stay in a similar dose-response fashion (10.5 days for patients with preoperative blood glucose >160 mg/dl, 9.4 days for patients with preoperative blood glucose 121–160 mg/dl, 6.9 days for patients with preoperative blood glucose 100–120 mg/dl, 5.2 days for patients with preoperative blood glucose <100 mg/dl, P < 0.001). Increased BMI was associated with length of ICU stay (P = 0.018) but not hospital stay. Male gender was not associated with length of ICU stay compared to female gender, but was associated with longer hospital stay compared to female gender (P = 0.003). Craniotomy cases, emergent procedures, traumatic cases, and occurrence of a postoperative complication all predicted longer stays in both the ICU (P < 0.001) and the hospital (P < 0.001). Increased age, chronic glucocorticoid use, diabetic status, use of insulin, and use of other diabetic medications were not associated with either length of ICU stay or hospital stay.
Table 2.
Analysis of variance between explanatory covariates and post-surgical hospital and intensive care unit stay among 918 patients undergoing neurosurgical intervention at the University of Michigan Hospitals
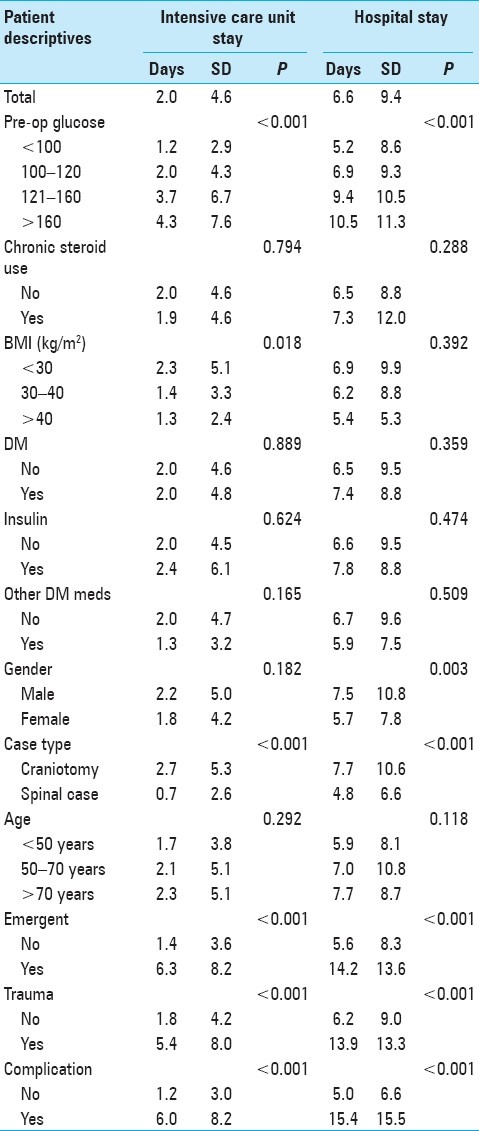
Table 3 shows a multivariable model of 30-day postoperative complication risk by preoperative blood glucose, adjusted for potential confounders. Preoperative blood glucose was associated with postoperative complication risk. Relative to blood glucose <100 mg/dl, odds of complications among patients with blood glucose 100–120 mg/dl were 1.6 (95% CI 0.9–2.6) and 2.1 (95% CI 1.2–3.4) among patients with blood glucose 121–160 mg/dl, and 2.2 (95% CI 1.1–4.5) among patients with blood glucose >160 mg/dl.
Table 3.
Multivariable logistic regression models of complication risk by preoperative blood glucose adjusted for potential confounders among 918 patients undergoing neurosurgical intervention at the University of Michigan Hospitals
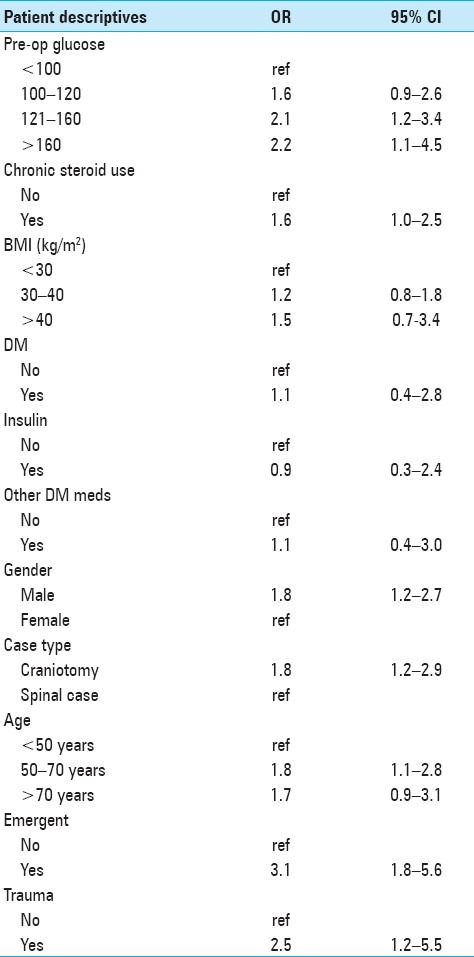
Table 4 shows ANCOVA between preoperative glucose and the other covariates of interest with respect to post-surgical hospital stay and length of ICU stay. After adjusting for the other covariates, preoperative blood glucose levels 121–160 mg/dl (P < 0.001) and >160 mg/dl (P < 0.001) were associated with prolonged ICU stay relative to blood glucose levels <100 mg/dl. Preoperative blood glucose levels 100–120 mg/dl were not associated with longer ICU stay (P = 0.839). Preoperative blood glucose 121–160 mg/dl (P < 0.001) and >160 mg/dl (P < 0.001) were also associated with increased length of hospital stay relative to levels <100 mg/dl. Preoperative blood glucose levels 100–120 mg/dl were not associated with length of hospital stay (P = 0.641). Finally, we performed a separate subgroup analysis excluding traumatic and emergent cases, and the observed relationship between preoperative hyperglycemia and postoperative complication rates was preserved across all glucose ranges by both univariate and multivariate analyses (data not shown).
Table 4.
Analysis of covariance between explanatory covariates and post-surgical hospital and intensive care unit stay among 918 patients undergoing neurosurgical intervention at the University of Michigan Hospitals
DISCUSSION
In a study of 918 consecutive neurosurgical patients at the University of Michigan Hospitals, we found that increased preoperative blood glucose was associated with higher risk for postoperative complications, longer hospital stays, and longer neurosurgical ICU stays. Moreover, increasing preoperative blood glucose predicted both complication risk and ICU and hospital stay in a dose–response fashion, such that incrementally higher blood glucose predicted incrementally higher complication risk and longer ICU and hospital stay.
The effects of preoperative blood glucose on general adult neurosurgical outcomes and complication risk are not well understood, even while a number of studies have investigated highly specific subsets of neurosurgical patients. In one study, for example, diabetic status was shown to be an independent risk factor for poor surgical outcome in repair of ossification of the posterior longitudinal ligament of the cervical spine.[18] In another, among diabetic patients undergoing image-guided stereotactic brain biopsy, serum blood glucose >200 mg/dl was predictive of biopsy-related morbidity relative to serum blood glucose <200 mg/dl.[24] Bilota et al. demonstrated a substantial difference in infection rates following emergent cerebral aneurysm clipping by level of glycemic control (80–220 mg/dl vs. 80–120 mg/dl).[4] However, other studies have shown no effect of peri-operative hyperglycemia on morbidity and mortality in the neurosurgical setting.[20,25,30] To our knowledge, no neurosurgical studies have investigated the effects of hyperglycemia on broader metrics of neurosurgical outcomes and complication risk among the general adult population. Furthermore, thresholds for clinically relevant hyperglycemia remain largely arbitrary within neurosurgery. Determination of validated thresholds is crucial, given the findings among patients from other surgical specialties which have shown that peri-operative hyperglycemia is associated with increased risk of surgical site infections,[10,12,27,31] and tight glycemic control has been shown to improve outcomes and reduce the risk for stroke and postoperative wound infections.[7–9,12,13,17,21–23,35]
There are a number of plausible explanations for our findings. It is possible that high preoperative blood glucose may reflect chronically poor glycemic control, predisposing patients to increased risk of complications seen in uncontrolled diabetics. Diabetic patients under good control would likely be protected against postoperative complications relative to uncontrolled diabetics.[10,12,27,31] Alternatively, acute hyperglycemia alone may be sufficient to worsen clinical outcomes. For instance, acute hyperglycemia is known to exacerbate ischemic brain damage in the absence of chronic hyperglycemia.[15] In addition, it remains plausible that acute hyperglycemia associates with a population with systematically worse health, for which we were unable to adjust in this study.
Physiologically, animal and human studies have elucidated several mechanisms by which hyperglycemia may effect pathology. As a result of hyperglycemia, the nonenzymatic glycosylation of proteins and lipids disrupts normal functioning, overproduction of superoxide anion by the mitochondrial electron transport chain increases oxidative stress, and induced cytokine release promotes inflammation.[2] These mechanisms are all associated with vascular damage and disruption of the immune system. The resultant poor microvascularization and immunocompromised state renders hyperglycemic patients particularly susceptible to postoperative complications.[1,14]
Traditional guidelines for control of blood glucose have recommended treatment in surgical patients to achieve glucose levels below 200 mg/dl.[20] Recent studies in cardiac surgery have suggested that more aggressive treatment of hyperglycemia aim for levels below 110 mg/dl to reduce the incidence of postoperative complications.[12,33] Our findings would support these calls for tighter glycemic control among patients undergoing neurosurgical intervention, as even blood glucose levels >120 mg/dl were associated with a doubling of risk for complications and increases in hospital and ICU duration of stay. However, the effects of tight glycemic control have not been studied prospectively among surgical patients in other subspecialties or in neurosurgery, and there are concerns regarding overly aggressive treatment of hyperglycemia, as potentially resultant hypoglycemia may also carry dangerous sequelae.[3]
As with any retrospective analysis, the results presented here are subject to several limitations. First, although we used multivariable techniques to adjust for potential confounders, it remains possible that there was residual confounding by unmeasured factors associated with both preoperative blood glucose and the outcomes of interest under study. Second, ascertainment of blood glucose levels was based on routine preoperative labs, and as there could be heterogeneity in the quality of preoperative data attained, especially by level of emergence, this is a substantial limitation. However, this is a limitation imposed by the retrospective nature of our analysis and was unavoidable. Third, since this study considered only patients having undergone neurosurgical intervention, our findings do not generalize to other surgical specialties. Fourth, we neither measured complication rates past 30 postoperative days, nor did we evaluate long-term clinical outcomes, further limiting the generalizability of our findings. Fifth, while we adjusted for emergent and traumatic cases, it remains plausible that elevated perioperative blood glucose may be a reflection of severity of underlying pathology for which we were unable to adjust. While severity of neurosurgical condition outside of the emergent population is not consistently known to correlate with elevated blood sugar in the non-diabetic patient, severity of underlying illness remains a potential source of bias in our study. Furthermore, it is theoretically possible that hyperglycemia may have differential effects on post-neurosurgical outcomes in the traumatic or emergent population, and future studies dedicated to this hypothesis may be sufficiently powered to detect such a relationship.
Despite these limitations, we feel this study provides several insights of clinical importance. The results presented here may help guide treatment strategies, particularly our finding that even relatively minor elevations in preoperative blood glucose may be associated with substantial increases in risk for postoperative complications and length of hospital and ICU stay. Closer monitoring and more rapid response for those patients most susceptible to adverse events may aid clinicians in managing developing complications, and thus perhaps reduce postoperative morbidity and mortality.
With regard to future research in this area, randomized clinical trials of the efficacy of glycemic control on post-neurosurgical outcomes among hyperglycemic and diabetic patients are needed. Additionally, it would be of substantial clinical utility to determine whether acutely high preoperative blood glucose is predictive of postoperative complications independent of the quality of long-term glycemic control. Research is also needed to determine whether preoperative hyperglycemia predisposes to all, or only a subset, of postoperative complications. Finally, future research may also consider the cellular mechanisms that may underlie the relation between preoperative hyperglycemia and post-neurosurgical complications observed here.
CONCLUSIONS
Preoperative blood glucose above 120 mg/dl predicted risk for postoperative complications, increased length of hospital stay, and increased length of neurosurgical ICU stay in a dose–response fashion following neurosurgical intervention. Tight glycemic control peri-operatively may lead to reduced risk for postoperative complications and more rapid recovery following neurosurgery. Randomized controlled trials of the effects of tight glycemic control on post-neurosurgical outcomes among hyperglycemics are in order.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/49/96071
Contributor Information
Matthew C. Davis, Email: mdav@med.umich.edu.
John E. Ziewacz, Email: jziewacz@med.umich.edu.
Stephen E. Sullivan, Email: ssulliva@med.umich.edu.
Abdulrahman M. El-Sayed, Email: ame2145@columbia.edu.
REFERENCES
- 1.Aragon D, Ring C, Covelli M. The influence of diabetes mellitus on postoperative infections. Crit Care Nurs Clin North Am. 2003;15:125–35. doi: 10.1016/s0899-5885(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 2.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia A, Cadman B, Mackenzie I. Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control. Anesth Analg. 2006;102:549–51. doi: 10.1213/01.ane.0000195236.73494.bf. [DOI] [PubMed] [Google Scholar]
- 4.Bilota F, Caramia R, Paoloni FP, Delfini R, Rosa G. Safety and efficacy of intensive insulin therapy in critical neurosurgical patients. Anesthesiology. 2009;110:611–9. doi: 10.1097/ALN.0b013e318198004b. [DOI] [PubMed] [Google Scholar]
- 5.Bochicchio GV, Joshi M, Bochicchio KM, Pyle A, Johnson SB, Meyer W, et al. Early hyperglycemic control is important in critically injured trauma patients. J Trauma. 2007;63:1353–8. doi: 10.1097/TA.0b013e31815b83c4. [DOI] [PubMed] [Google Scholar]
- 6.Browne J, Cook C, Pietrobon R, Bethel MA, Richardso WJ. Diabetes and early postoperative outcomes following lumbar fusion. Spine. 2007;32:2214–9. doi: 10.1097/BRS.0b013e31814b1bc0. [DOI] [PubMed] [Google Scholar]
- 7.Capes S, Hunt D, Malmberg K, Pathak P, Gerstein H. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke. 2001;32:2426–32. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 8.Capes S, Hunt D, Malmberg K, Pathak P, Gerstein H. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet. 2000;355:773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 9.Carr J, Sellke F, Fey M, Doyle MJ, Krempin JA, de la Torre R, et al. Implementing tight glucose control after coronary artery bypass surgery. Ann Thorac Surg. 2005;80:902–9. doi: 10.1016/j.athoracsur.2005.03.105. [DOI] [PubMed] [Google Scholar]
- 10.Estrada CA, Young JA, Nifong LW, Chitwood WR., Jr Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;75:1392–9. doi: 10.1016/s0003-4975(02)04997-4. [DOI] [PubMed] [Google Scholar]
- 11.Friedman N, Sexton D, Connelly S, Kaye KS. Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol. 2007;28:1060–5. doi: 10.1086/519864. [DOI] [PubMed] [Google Scholar]
- 12.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: The Portland Diabetic Project. Endocrine Pract. 2004;10(Suppl 2):21–33. doi: 10.4158/EP.10.S2.21. [DOI] [PubMed] [Google Scholar]
- 13.Goodson W, Hunt T. Wound healing and the diabetic patient. Surg Gynecol Obstet. 1979;149:600–8. [PubMed] [Google Scholar]
- 14.Homer-Vanniasinkam S. Surgical site and vascular infections: Treatment and prophylaxis. Int J Infect Dis. 2007;11(Suppl 1):S17–22. doi: 10.1016/S1201-9712(07)60003-4. [DOI] [PubMed] [Google Scholar]
- 15.Ittichaikulthol W, Lekprasert V, Pausawasdi S, Suchartwatnachai P. Effect of intraoperative fluid on blood glucose level in neurosurgery. J Med Assoc Thai. 1997;80:461–5. [PubMed] [Google Scholar]
- 16.Kao LS, Meeks D, Moyer VA, Lally KP. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev. 2009:CD006806. doi: 10.1002/14651858.CD006806.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King L. Impaired wound healing in patients with diabetes. Nurs Stand. 2001;15:39–45. doi: 10.7748/ns2001.06.15.38.39.c3039. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Jiang L, Dai L. A review of prognostic factors for surgical outcome of ossification of the posterior longitudinal ligament of cervical spine. Eur Spine J. 2008;17:1277–88. doi: 10.1007/s00586-008-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malone D, Genuit T, Tracy J, Gannon C, Napolitano LM. Surgical site infections: Reanalysis of risk factors. J Surg Res. 2002;103:89–95. doi: 10.1006/jsre.2001.6343. [DOI] [PubMed] [Google Scholar]
- 20.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. [PubMed] [Google Scholar]
- 21.Marchant M, Viens N, Cook C, Vail T, Bolognesi M. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91:1621–9. doi: 10.2106/JBJS.H.00116. [DOI] [PubMed] [Google Scholar]
- 22.McAlister F, Majumdar S, Blitz S, Rowe B, Romney J, Marrie T. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28:810–5. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 23.McAlister F, Man J, Bistritz L, Amad H, Tandon P. Diabetes and coronary artery bypass surgery: An examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26:1518–24. doi: 10.2337/diacare.26.5.1518. [DOI] [PubMed] [Google Scholar]
- 24.McGirt MJ, Woodworth GF, Coon AL, Frazier JM, Amundson E, Garonzik I, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: A risk assessment of 270 cases. J Neurosurg. 2005;102:897–901. doi: 10.3171/jns.2005.102.5.0897. [DOI] [PubMed] [Google Scholar]
- 25.Patel N, Bagan B, Vadera S, Maltenfort M, Deutsch H, Vaccaro A, et al. Obesity and spine surgery: Relation to perioperative complications. J Neurosurg Spine. 2007;6:291–7. doi: 10.3171/spi.2007.6.4.1. [DOI] [PubMed] [Google Scholar]
- 26.Patil C, Veeravagu A, Shivanand P. Craniotomy for resection of meningioma in the elderly: A multicentre, prospective analysis from the National Surgical Quality Improvement Program. J Neurol Neurosurg Psychiatry. 2010;81:502–5. doi: 10.1136/jnnp.2009.185074. [DOI] [PubMed] [Google Scholar]
- 27.Pomposelli JJ, Baxter JK, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 28.Singleton R, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–73. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- 29.Simpson JM, Silveri CP, Balderston RA, Simeone FA, An HS. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993;75:1823–9. doi: 10.2106/00004623-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 30.The DECODE Study Group: Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–21. [PubMed] [Google Scholar]
- 31.Trick WE, Scheckler WE, Tokars JI, Jones KC, Reppen ML, Smith EM, et al. Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119:108–14. doi: 10.1016/s0022-5223(00)70224-8. [DOI] [PubMed] [Google Scholar]
- 32.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. The Finnish diabetes prevention study group: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 34.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 35.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–8. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]


