Table 1.
Lewis Acid Promoted Intermolecular Diels-Alder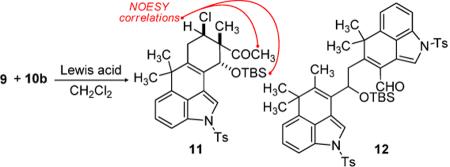
| Entry | Lewis acid | temp, time | 11:12:8[a] | yield (11)[b] |
|---|---|---|---|---|
| 1 | TMSOTf | −78 °C, 2 h | 1:0:1 | 18% |
| 2 | TiCl4 | −20 °C, 0.5 h | 1:3:1 | 24% |
| 3 | Ti(iOPr)4 | 0 °C, 12 h | 0:0:1 | – |
| 4 | Et2AlCl | 0 °C, 12 h | 0:0:1 | – |
| 5 | (CH3)3Al | 0 °C, 12 h | 0:0:1 | – |
| 6 | EtAlCl2 | −20 °C, 1 h | 3:1:0 | 36% |
| 7 | EtAlCl2[c] | −78 → −20 °C, 3 h | 13:1:1 | 59% |
Determined by analysis of the crude reaction mixture by 1H NMR.
Isolated yield.
Substitution of CH2Cl2 for toluene as the solvent provided 11 in 54% yield.
