Table 2.
Rhodium-catalyzed O—H insertion
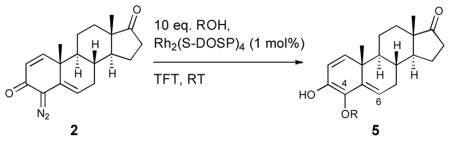 | |||||
|---|---|---|---|---|---|
| entry | R | product | time (h) | C-4:C-6a | yield (%)b |
| 1 | -CH2CH3 | 5a | 6 | >95:5 | 61 |
| 2 | -CH3 | 5b | 6 | 76:24 | 49 |
| 3 | -CH2Ph | 5c | 12 | >95:5 | 52 |
| 4 | -(CO)CH3 | 5d | 8 | 92:8 | 68 |
| 5 | -(CO)CH2CH3 | 5e | 6 | 88:12 | 55 |
| 6 | -(CO)CH2Ph | 5f | 16 | >95:5 | 41 |
Ratio determined by 1H NMR spectroscopy of the crude reaction mixture. All reactions were run at a concentration of 0.15 M with respect to 2.
Isolated yield of the C-4 regioisomer
