Abstract
BACKGROUND
This study evaluated the predictive value of serum and follicular fluid (FF) concentrations of anti-Müllerian hormone (AMH) with respect to treatment outcome variables in an IVF cycle.
METHODS
A retrospective analysis was performed with data from 731 normogonadotrophic women undergoing controlled ovarian stimulation after stimulation with highly purified menotrophin (HP-hMG) or rFSH following a long GnRH agonist protocol.
RESULTS
In both treatment groups, the serum AMH concentration at the start of the stimulation was significantly (P < 0.001) positively correlated with the serum levels of estradiol (HP-hMG: r = 0.45; rFSH: r = 0.55), androstenedione (HP-hMG: r = 0.50; rFSH: 0.49) and total testosterone (HP-hMG: r = 0.40; rFSH: r = 0.36) at the end of the stimulation as well as the number of oocytes retrieved (HP-hMG: r = 0.48; rFSH: r = 0.62), the AMH concentration in FF (HP-hMG: r = 0.55; rFSH: 0.61) and the serum progesterone concentration (HP-hMG: r = 0.39; rFSH: r = 0.50) at oocyte retrieval. For both treatments, serum AMH at the start of the stimulation was a good predictor of the need to increase or decrease the gonadotrophin dose on stimulation day 6 and of ovarian response below (<7 oocytes) or above (>15 oocytes) the target. No significant relationships were observed between serum AMH and embryo quality or ongoing pregnancy.
CONCLUSION
The serum AMH concentration at the start of the stimulation in IVF patients down-regulated with GnRH agonist in the long protocol revealed a positive relationship with ovarian response to gonadotrophins in terms of oocytes retrieved and accompanying endocrine response. AMH is a good predictor of the need for gonadotrophin-dose adjustment on stimulation day 6 for patients with a fixed starting dose, but a poor predictor of embryo quality and pregnancy chances in individual patients.
Keywords: AMH, gonadotrophins, ovarian response, prediction, IVF cycle
Introduction
Anti-Müllerian hormone (AMH) is a glycoprotein belonging to the transforming growth factor-β superfamily (Cate et al, 1986). In women, AMH is produced solely by the ovarian granulosa cells. Its expression is low in primary follicles but increases to maximal levels in pre-antral and small antral follicles (up to 6–7 mm in diameter). AMH expression gradually declines as follicles increase further in size and ultimately becomes undetectable at a stage where FSH-dependent follicular growth has been initiated (Weenen et al., 2004). AMH is not expressed in atretic follicles.
AMH plays a role in the control of follicle growth via paracrine and autocrine effects. Both in vitro and in vivo studies have shown that AMH inhibits the recruitment of resting follicles from the primordial follicle pool (Durlinger et al., 1999; Carlsson et al., 2006) and AMH decreases the sensitivity of small antral follicles to FSH (Durlinger et al., 2001). The serum level of AMH is a marker of the ovarian reserve; the number of antral follicles has been shown to correlate more strongly with serum AMH than with the serum level of FSH (Fanchin et al., 2003a). Moreover, AMH appears to be the earliest endocrine marker of ovarian aging (de Vet et al., 2002).
The serum AMH level shows only minimal fluctuations during the menstrual cycle (La Marca et al., 2006), reflecting the continuous non-cyclic growth of pre-antral and small antral follicles (independent of FSH). Furthermore, the serum level of AMH displays a high inter-cycle reproducibility. Consequently, only a single blood sample is sufficient for a reliable assessment of the ovarian reserve, compared with three measurements for FSH (Fanchin et al., 2005). Serum AMH has also been shown to demonstrate less intra- and inter-cycle variation than the antral follicle count (AFC), suggesting that the AMH constitutes a more reliable and robust marker of ovarian reserve than the actual AFC assessment (van Disseldorp et al., 2010). An association between basal AMH and ovarian response to gonadotrophin was first reported by Seifer et al. (2002). Since then, a number of studies have claimed AMH to be superior to the age of the patient and measurements of FSH, estradiol and inhibin B on cycle day 2–3 in predicting oocyte yield in IVF (La Marca et al., 2010).
A few studies have assessed the effect of controlled ovarian stimulation (COS) on AMH secretion by the ovary. Unlike normal menstrual cycles where the serum AMH concentration does not change significantly, serum AMH levels have been demonstrated to decrease gradually during the follicular phase in both pituitary-desensitized GnRH agonist cycles (Fanchin et al., 2003b; La Marca et al., 2004) and GnRH antagonist cycles (Lee et al., 2010). The steady decrease in AMH levels during the follicular phase of the COS cycle has been suggested to reflect the change in follicle distribution, i.e. reduced numbers of small antral follicles and increased numbers of enlarged follicles, in response to the FSH stimulation (Fanchin et al., 2003b). In addition, FSH and estradiol have been reported to suppress AMH secretion (Baarends et al., 1995; Pellatt et al., 2007), which may contribute to the decrease in AMH concentrations during COS.
Serum level of AMH has been demonstrated to be a good prognostic marker for poor ovarian response, cycle cancellation and hyper-response to COS. Importantly, a recent large prospective (but non-randomized) study suggested that an AMH-based approach to individualize treatment strategies for COS may result in a reduced risk of hyper-response with maintained pregnancy rates (Nelson et al., 2009). It should be noted that most of the studies investigating the prognostic value of AMH in predicting ovarian response and/or outcome in ART have only included rFSH-treated cycles. To date, no studies have compared the usefulness of AMH measurements when different gonadotrophin preparations are used for stimulation.
Although it is recognized that embryo morphology grading is only a weak predictor of clinical outcome, one study has found a positive relationship between serum AMH after stimulation and embryo morphology (Silberstein et al., 2006), while others have demonstrated no correlation between basal serum AMH and embryo quality (Ebner et al., 2006; Smeenk et al., 2007; Lie Fong et al., 2008). Likewise, some studies have found serum AMH to be a predictor of pregnancy (Hazout et al., 2004; Eldar-Geva et al., 2005; Lekamge et al., 2007; Elgindy et al., 2008), while other studies (van Rooij et al., 2002; Peñarrubia et al., 2005; Fiçicioglu et al., 2006; Smeenk et al., 2007; Kwee et al., 2008) and recent meta-analyses (Broer et al., 2009, 2010) could only attribute a poor predictive value to serum AMH for pregnancy after ART. Indeed the vast majority of studies have concluded that AMH is not an independent prognostic marker for pregnancy outcome (Nelson et al., 2007; La Marca et al., 2010), suggesting that the basal serum AMH level is a marker of quantitative rather than qualitative aspects of the ovarian reserve. There are only sparse data on AMH in the follicular fluid (FF). However, it has been suggested that AMH in FF, but not in serum, is positively associated with embryo implantation and clinical pregnancy rate (Fanchin et al., 2007).
The aim of the present retrospective study was to evaluate the associations between serum and FF AMH levels and ovarian response (including endocrine variables), embryo quality as well as ongoing pregnancy rates after stimulation with two different gonadotrophin preparations, highly purified menotrophins (HP-hMG) and rFSH, in GnRH agonist cycles for IVF. Also, this study compared the serum AMH dynamics during COS between the two treatment groups.
Materials and Methods
Study population
This study is a retrospective analysis of data derived from a randomized, active-controlled, assessor-blind, multicentre, multinational trial; the details of which have been described previously (Nyboe Andersen et al., 2006). The trial compared pregnancy rates in patients (n = 731) undergoing IVF after stimulation with HP-hMG (Menopur; Ferring Pharmaceuticals A/S, Copenhagen, Denmark) or rFSH (follitropin alfa, Gonal-F; Merck Serono, Geneva, Switzerland). Main inclusion criteria were patients with major indications for IVF such as tubal factor infertility or unexplained infertility including endometriosis stage I/II or partners with mild semen abnormalities not requiring ICSI, an age of at least 21 but not more than 37 years, a body mass index (BMI) of 18–29 kg/m2, FSH within normal limits (1–12 IU/l), regular menstrual cycles of 21–35 days which were presumed to be ovulatory and a willingness to accept transfer of one or two embryos. The randomization of patients to treatment were stratified by age (<35 and 35–37 years). Patients with polycystic ovary syndrome, endometriosis stage III/IV or partners with severe male factors requiring ICSI were excluded as poor responders; the study population consisted of infertile women with favorable prognosis.
Study protocol
Patients underwent COS following down-regulation with a GnRH agonist in a long protocol. Pituitary suppression with triptorelin acetate, 0.1 mg/day subcutaneously (Decapeptyl; Ferring Pharmaceuticals A/S), was initiated 5–7 days before the estimated start of next menses and continued until the end of gonadotrophin administration. Prior to start of ovarian stimulation, the antral follicle count (AFC; follicles >2 mm) was recorded by transvaginal ultrasound (TVU) of the ovaries by one or more operators at the clinics and follicular development was monitored after 5 days of treatment and thereafter at least every 2 days. Stimulation with HP-hMG or rFSH was started at a dose of 225 IU/day for the first 5 days and was followed by individual dose-adjustments according to the patient's follicular response as exclusively measured by TVU. The daily dose could either be increased or decreased by 75 IU per adjustment and not changed more frequently than every fourth day. Recombinant hCG (choriongonadotrophin alfa, Ovitrelle; Merck Serono), 250 μg subcutaneously, was used to induce final follicular maturation when three or more follicles of ≥17 mm in diameter were observed and was administered 36 ± 2 h before planned oocyte retrieval. Coasting was not allowed. The target for the ovarian stimulation was set to be 7–15 oocytes at retrieval as 7 or more oocytes are considered to give reasonable chances (∼25%) of pregnancy and the risk of developing moderate/severe ovarian hyperstimulation syndrome (OHSS) is low in patients with ≤15 oocytes (Arce et al., 2005). Criteria for cycle cancellation at the day of hCG administration were either inability to reach the hCG criterion or >25 follicles with a diameter of ≥10 mm.
A top-quality embryo (TQE) was defined as four to five cells on Day 2, seven or more cells on Day 3, equally-sized blastomeres and ≤20% fragmentation on Day 3 and no multinucleation. The transfer of one or two embryos was done on Day 3 after oocyte retrieval according to local practise within any country specific regulations. Vaginal progesterone gel of 90 mg/day 8% (Crinone; Merck Serono) for luteal support was given from the day of embryo transfer until the confirmation of clinical pregnancy (5–6 weeks after embryo transfer) or negative serum hCG test (13–15 days after embryo transfer).
Collection and handling of serum
Blood samples for AMH analysis were drawn on Days 1 and 6 and on the last day of stimulation as well as the day of oocyte retrieval. The sample on Day 1 was taken before the start of gonadotrophin administration and the samples on Day 6 and last stimulation day were collected at least 8 h after the previous gonadotrophin dose. Blood samples were centrifuged for 10 min at 1800g. Serum was stored individually at −18°C or colder at the clinic for a maximum of 2 weeks before transfer to −70°C and subsequent analysis at a central laboratory. The sera were only thawed once.
Collection and handling of FF
AMH measurements in FF were performed in those patients who had oocyte retrieval. Fluid was collected from one follicle of ≥17 mm from which an oocyte was retrieved. Fluid was preferably collected from the first follicle aspirated and from follicles where flushing had not been performed. The fluid was centrifuged for 10 min at 1000g and the supernatant was stored under the same conditions as serum. Fluids that were found to be contaminated by red blood cells or flushing medium were not included in the analysis.
Analytical methods for the variables measured in serum and FF
Serum and FF AMH analysis was performed batch wise in a single laboratory (hormone laboratory at Universitair Ziekenhuis, Brussels) to minimize variability. AMH was measured with the Immunotech Beckman Coulter AMH ELISA kit (A11893). The intra-assay and inter-assay coefficients of variation were <9.5%. Functional sensitivity of the assay was 2.5 pmol/l (1 ng/ml = 7.14 pmol/l).
Serum was analysed for other endocrine variables by a central laboratory using electrochemiluminescence immunoassays (FSH, LH, hCG), chemiluminescent immunometric assays (estradiol, progesterone, SHBG) and radioimmunoassays (androstenedione, total testosterone). The sensitivity [and total imprecision (coefficient of variation, CV)] of the validated analytical methods were as follows: FSH <0.1 IU/l (<6%), LH <0.1 IU/l (<6%), hCG <0.1 IU/l (<8%), estradiol 55 pmol/l (10%), progesterone 0.6 nmol/l (<8%), SHBG 0.02 nmol/l (10%), androstenedione 0.08 nmol/l (10%) and total testosterone 0.17 nmol/l (5%).
FF was analysed for endocrine variables by a central laboratory using electrochemiluminescence assays (FSH, LH, hCG, estradiol, progesterone, SHBG), radioimmunoassays (androstenedione, total testosterone, cortisol, cortisone) and ELISA (inhibin A, VEGF, IGF-1). The sensitivity (and total imprecision, CV) were as follows: FSH <0.1 IU/l (<5%), LH 0.1 IU/l (<5%), hCG <0.1 IU/l (<8%), estradiol 18 pmol/l (<5%), progesterone 0.095 nmol/l (<5%), SHBG 0.35 nmol/l (<5%), androstenedione 0.13 nmol/l (<12%), total testosterone 0.1 nmol/l (<8%), cortisol 20 nmol/l (<8%), cortisone 4 nmol/l (<10%), inhibin A 1.0 ng/l (9%), VEGF <9.0 ng/l (7%) and IGF-1 0.105 nmol/l (10%).
Statistical analysis
The study cohort comprised all patients with AMH measurements at the start of the stimulation. The endocrine variables are reported using median and inter-quartile range (IQR) due to non-symmetric distributions. Spearman's rank correlation coefficient was used to quantify the association between pairs of variables. Due to the high number of observations, a large proportion of the calculations lead to significant correlations. To avoid multiplicity, mainly highly significant (P < 0.001) clinically relevant (r > 0.30) correlations were considered of interest.
The relationships between serum AMH at the start of the stimulation and baseline, stimulation and outcome characteristics were evaluated using regression models. In all models, AMH was included as covariate and treatment was included as a factor. ANCOVA models were utilized for continuous variables, while categorical outcomes were modelled using logistic regression for binary outcomes and proportional odds models for ordinal outcomes. Receiver operating characteristic (ROC) curves were used to evaluate the ability of AMH to discriminate between relevant outcomes of stimulation. ROC curves were based on simple logistic regression models using AMH as a covariate.
Results
Serum AMH at the start of the stimulation
Amongst the 731 patients who initiated COS, 623 patients had serum AMH measurements on stimulation day 1: 314 patients treated with HP-hMG and 309 patients treated with rFSH. There were no apparent differences between the two treatment groups with respect to demographics, baseline characteristics and serum hormone concentrations at the start of the stimulation (Table I).
Table I.
Patient demographics, baseline characteristics and serum endocrine concentrations at the start of the stimulation.
| Variable | Alla (n = 623) | HP-hMGa (n = 314) | rFSHa (n = 309) |
|---|---|---|---|
| Baseline | |||
| Age (years), mean (SD) | 30.8 (3.3) | 30.8 (3.2) | 30.8 (3.4) |
| Weight (kg), mean (SD) | 62.1 (8.4) | 62.8 (8.5) | 61.3 (8.3) |
| BMI (kg/m2), mean (SD) | 22.3 (2.6) | 22.5 (2.7) | 22.1 (2.6) |
| Duration of infertility (years), mean (SD) | 3.9 (2.2) | 3.9 (2.3) | 3.9 (2.2) |
| Primary cause of infertility | |||
| Tubal infertility, n (%) | 213 (34%) | 116 (37%) | 97 (31%) |
| Mild male factor, n (%) | 59 (9%) | 29 (9%) | 30 (10%) |
| Other (incl. endometriosis I/II), n (%) | 65 (10%) | 31 (10%) | 34 (11%) |
| Unexplained infertility, n (%) | 286 (46%) | 138 (44%) | 148 (48%) |
| Duration of GnRH agonist before the start of stimulation (days) | 15.1 (4.0) | 15.1 (4.1) | 15.1 (3.9) |
| Day 1 of stimulation | |||
| Mean ovarian volume (ml) | 4.3 (3.0, 5.9) | 4.4 (3.1, 5.9) | 4.3 (2.9, 5.9) |
| AFC | 10 (7, 14) | 10 (7, 14) | 10 (7, 14) |
| 21–29 years | 11 (7, 16); n = 209 | 11 (8, 16); n = 105 | 10 (7, 16); n = 104 |
| 30–34 years | 10 (7, 14); n = 316 | 9 (7, 14); n = 165 | 10 (7, 14); n = 151 |
| 35–37 years | 10 (7, 14); n = 94 | 10 (7, 14); n = 42 | 9 (7, 14); n = 52 |
| FSH (IU/l) | 3.7 (2.9, 4.7) | 3.7 (2.9, 4.7) | 3.8 (3.0, 4.7) |
| LH (IU/l) | 1.9 (1.4, 2.7) | 1.8 (1.3, 2.7) | 2.0 (1.5, 2.6) |
| Progesterone (nmol/l) | 1.2 (0.9, 1.5) | 1.2 (0.9, 1.5) | 1.1 (0.9, 1.5) |
| Androstenedione (nmol/l) | 4.1 (3.1, 5.4) | 4.3 (3.1, 5.5) | 3.9 (3.0, 5.1) |
| Total testosterone (nmol/l) | 0.6 (0.5, 0.9) | 0.6 (0.5, 0.9) | 0.6 (0.4, 0.8) |
| SHBG (nmol/l) | 55 (42, 71) | 56 (40, 72) | 55 (42, 69) |
| Free androgen index | 1.1 (0.7, 1.7) | 1.2 (0.8, 1.8) | 1.1 (0.7, 1.7) |
| AMH (pmol/l) | 26.0 (16.8, 37.4) | 25.5 (17.2, 36.1) | 26.6 (16.4, 37.9) |
| 21–29 years | 30.7 (20.1, 44.8); n = 210 | 30.7 (20.7, 46.6); n = 106 | 30.7 (18.7, 41.4); n = 104 |
| 30–34 years | 25.1 (16.3, 37.2); n = 317 | 24.3 (16.3, 34.0); n = 165 | 26.5 (16.4, 38.9); n = 152 |
| 35–37 years | 19.0 (11.9, 27.9); n = 96 | 19.7 (13.3, 27.9); n = 43 | 18.4 (11.5, 27.9); n = 53 |
Values are median (IQR) unless otherwise indicated.
aPatients with AMH measurement at the start of stimulation.
In the total cohort, the median (IQR) concentration of serum AMH at the start of the stimulation after two weeks of pituitary down-regulation with GnRH agonist was 26.0 (16.8, 37.4) pmol/l (Table I). The two treatment groups were similar with respect to median basal AMH in the various age categories. There was a significant (P < 0.001) negative correlation between serum AMH at the start of the stimulation and age, even though the correlation coefficient was relatively small (r = −0.25). The serum concentration of AMH was positively correlated with AFC (r = 0.35, P < 0.001), while the correlation between age and AFC was weak (r = −0.08, P = 0.046). Also, only weak correlations (r ≤ 0.15) were observed at the start of the stimulation between serum AMH and serum concentrations of FSH, LH, estradiol, progesterone, androstenedione, SHBG, total testosterone and free androgen index.
Serum AMH during stimulation
The serum AMH concentration decreased gradually during COS and showed similar dynamic changes in both treatment groups, although a significantly (P < 0.001) larger reduction was noted during stimulation with rFSH than with HP-hMG. In the rFSH group, the median AMH (IQR) concentration was decreased by 30% to 18.6 (12.8, 27.5) pmol/l on Day 6 of stimulation and by 55% to 11.9 (8.1, 16.9) pmol/l on the last day of stimulation, while the median AMH concentration was decreased by 16% to 21.4 (14.9, 32.7) pmol/l on Day 6 and 46% to 13.7 (10.3, 19.6) pmol/l on the last day of stimulation in the HP-hMG group. The serum concentration of AMH on stimulation day 1 was significantly (P < 0.001) correlated with the serum concentration on Day 6 and on the last day of stimulation in both treatment groups (HP-hMG group: r = 0.91 and 0.82, respectively; rFSH group: r = 0.93 and 0.84, respectively). The magnitude of the decreases in serum AMH from baseline to stimulation day 6 as well as during the whole stimulation period was correlated (P < 0.001) with the number of follicles ≥10 mm on Day 6 and on the last stimulation day (HP-hMG group: r = 0.33 and 0.51, respectively; rFSH group: r = 0.38 and 0.57, respectively).
Serum AMH association with endocrine values
Serum concentrations of estradiol and androstenedione on Day 6 of stimulation were found to be significantly (P < 0.001) positively correlated with the serum AMH concentrations at the start of stimulation (HP-hMG group: r = 0.38 and 0.31, respectively; rFSH group: r = 0.58 and 0.34, respectively). Also, significant (P < 0.001) positive correlations were observed between serum AMH concentrations at the start of stimulation and concentrations, on the last stimulation day and on the day of oocyte retrieval respectively, of serum estradiol (HP-hMG group: r = 0.45 and 0.49, respectively; rFSH group: r = 0.55 and 0.53, respectively), androstenedione (HP-hMG group: r = 0.50 and 0.52, respectively; rFSH group: r = 0.49 and 0.49, respectively), and total testosterone (HP-hMG group: r = 0.40 and 0.44, respectively; rFSH group: r = 0.36 and 0.39, respectively). The serum progesterone concentration at oocyte retrieval was also significantly (P < 0.001) correlated with serum AMH measured at the start of the stimulation (HP-hMG group: r = 0.39; rFSH group: r = 0.50). Correlations between serum AMH at the start of the stimulation and all other endocrine variables in serum measured on Day 6 or at the end of the stimulation were <0.30. Similar relationships between AMH and endocrine variables were observed when using AMH concentrations on Day 6 or at the end of stimulation instead of AMH at the start of the stimulation.
Serum AMH association with ovarian response
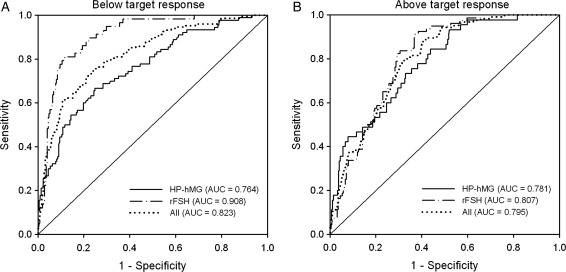
Oocyte retrieval was performed for 95% of the patients with AMH measurement at stimulation Day 1: 299 and 294 patients in the HP-hMG and rFSH groups, respectively. The median (IQR) number of oocytes retrieved was significantly (P < 0.001) higher in the rFSH group compared with the HP-hMG group: 11 (8, 16) versus 9 (6, 13). The serum concentrations of AMH on stimulation days 1, 6 and last day were all significantly (P < 0.001) positively correlated with the number of oocytes retrieved. In both treatment groups, the correlations were stronger for basal AMH (HP-hMG: r = 0.48; rFSH: r = 0.62) than for AMH on Day 6 (HP-hMG: r = 0.42; rFSH: r = 0.58) or AMH on the last day (HP-hMG: r = 0.35; rFSH: r = 0.48). Compared with AMH, AFC was less strongly correlated with the number of oocytes retrieved (HP-hMG group: r = 0.19, P = 0.001; rFSH group: r = 0.36, P < 0.001). ROC analyses were performed to assess the predictive value of serum AMH on stimulation day 1 for estimation of oocyte retrievals below and above the target, respectively. The predictive performance of the two models was high in both treatment groups (below the target: 0.764 and 0.908 for HP-hMG and rFSH, respectively; above the target: 0.781 and 0.807 for HP-hMG and rFSH, respectively; Fig. 1). The corresponding ROC values for AFC were significantly (P < 0.05) lower (below the target: 0.618 and 0.699 for HP-hMG and rFSH, respectively; above the target: 0.630 and 0.675 for HP-hMG and rFSH, respectively) than for AMH.
Figure 1.
ROC curve analysis showing the predictive value of serum AMH at the start of the stimulation for the estimation of number of oocytes at retrieval below (<7) (A) and above (>15) the target (B) after COS in patients treated with HP-hMG or rFSH in the long GnRH agonist protocol. The diagonal line is the reference line of no discrimination (AUC = 0.5). Cut-off values for response below the target were 21.2 pmol/l for HP-hMG (sens. 66.7%, spec. 75.2%) and 16.4 pmol/l for rFSH (sens. 81.0%, spec. 88.3%). The cut-off values for response above the target were 29.8 pmol/l for HP-hMG (sens. 73.3%, spec. 67.0%) and 29.5 pmol/l for rFSH (sens. 82.5%, spec. 70.4%).
Baseline, stimulation and outcome characteristics according to AMH percentiles
The whole patient population was stratified according to the 25, 50 and 75th percentiles of serum AMH concentration at the start of stimulation. Baseline characteristics, stimulation characteristics, embryo quality and pregnancy rates per AMH quartile for all patients and for each treatment group are presented in Table II. There were significant (P < 0.001) differences between quartiles for all outcome variables except for the number of TQEs, when accounting for the number of oocytes retrieved, and the ongoing pregnancy rate. In both the HP-hMG and rFSH treatment groups, patients with lower AMH concentrations required higher total gonadotrophin doses and more treatment days to reach the hCG criteria than patients with higher AMH concentrations. Despite this, fewer oocytes were retrieved and the proportion below the stimulation target was higher in patients with lower AMH concentrations compared with patients with higher AMH concentrations. In contrast, cycle cancellations due to excessive response and cycles with ovarian response above the target and moderate/severe early OHSS were most frequent in patients in the highest AMH quartile.
Table II.
Baseline, stimulation and outcome variables grouped by AMH quartiles at the start of the stimulation.
| Variable | AMH quartiles |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <P25a |
P25–P50b |
>P50–P75c |
>P75d |
P-value (AMH) | P-value (treatment) | |||||||||
| All (n = 155) | HP-hMG (n = 73) | rFSH (n = 82) | All (n = 156) | HP-hMG (n = 86) | rFSH (n = 70) | All (n = 157) | HP-hMG (n = 79) | rFSH (n = 78) | All (n = 155) | HP-hMG (n = 76) | rFSH (n = 79) | |||
| Serum AMH at the start of stimulation (pmol/l)e | ||||||||||||||
| MeanIQR | 12.4 (10.1, 15.2) | 13.5 (10.6, 15.4) | 12.1 (9.3, 14.8) | 21.3 (18.5, 23.6) | 21.2 (18.8, 23.3) | 21.6 (18.3, 23.7) | 31.7 (28.8, 34.3) | 30.6 (28.7, 33.6) | 33.2 (28.9, 35.1) | 50.3 (41.1, 60.6) | 50.3 (42.3, 61.0) | 50.3 (40.9, 60.2) | ||
| Age (years)f | 32.0 (3.4) | 32.2 (3.3) | 31.8 (3.5) | 30.8 (3.3) | 30.6 (3.3) | 31.1 (3.2) | 30.5 (3.2) | 30.6 (3.0) | 30.3 (3.5) | 29.9 (3.0) | 29.9 (2.9) | 30.0 (3.1) | <0.001g | 0.952g |
| AFCe | 7 (5, 10) | 7 (5, 11) | 7 (4, 9) | 10 (7, 14) | 10 (7, 13) | 12 (7, 14) | 11 (8, 16) | 11 (8, 16) | 11 (8, 16) | 11 (8, 18) | 12 (8, 20) | 11 (8, 16) | <0.001g | 0.868g |
| FSH at the start of stimulation (IU/l)e | 4.0 (3.0, 5.1) | 3.6 (3.0, 5.0) | 4.2 (3.0, 5.2) | 3.8 (3.0, 4.6) | 4.0 (3.2, 4.8) | 3.4 (3.0, 4.4) | 3.7 (3.1, 4.6) | 3.7 (3.1, 4.4) | 3.7 (3.0, 4.7) | 3.4 (2.7, 4.4) | 3.2 (2.6, 4.4) | 3.6 (2.8, 4.4) | 0.084g | 0.382g |
| Dose adjustment on stimulation day 6 | <0.001h | 0.034h | ||||||||||||
| Decrease, n (%) | 2 (1) | 1 (1) | 1 (1) | 7 (4) | 4 (5) | 3 (4) | 10 (6) | 6 (8) | 4 (5) | 37 (24) | 13 (17) | 24 (30) | ||
| No change, n (%) | 75 (48) | 34 (47) | 41 (50) | 104 (67) | 56 (65) | 48 (69) | 123 (78) | 55 (70) | 68 (87) | 104 (67) | 55 (72) | 49 (62) | ||
| Increase, n (%) | 78 (50) | 38 (52) | 40 (49) | 45 (29) | 26 (30) | 19 (27) | 24 (15) | 18 (23) | 6 (8) | 14 (9) | 8 (11) | 6 (8) | ||
| Gonadotrophin dose on stimulation day 6 (IU)f | 262 (40) | 263 (40) | 261 (40) | 243 (39) | 244 (40) | 242 (39) | 232 (34) | 236 (40) | 227 (27) | 214 (42) | 220 (39) | 208 (43) | <0.001g | 0.047g |
| Number of treatment daysf | 11.2 (2.4) | 11.5 (2.6) | 10.9 (2.1) | 10.2 (1.5) | 10.1 (1.6) | 10.3 (1.5) | 10.2 (1.4) | 10.5 (1.5) | 9.9 (1.2) | 9.8 (1.5) | 10.0 (1.5) | 9.7 (1.5) | <0.001g | 0.042g |
| Total gonadotrophin dose (IU)f | 2874 (942) | 2975 (1022) | 2783 (860) | 2452 (578) | 2432 (620) | 2476 (526) | 2341 (467) | 2452 (554) | 2229 (325) | 2130 (426) | 2199 (438) | 2063 (406) | <0.001g | 0.025g |
| Cycle cancelled | ||||||||||||||
| Poor response, n (%) | 13 (8) | 7 (10) | 6 (7) | 2 (1) | 2 (2) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | <0.001h | 0.127h |
| High response, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 1 (1) | 7 (5) | 3 (4) | 4 (5) | <0.001h | 0.278h |
| Early OHSS (moderate/severe), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 7 (5) | 3 (4) | 4 (5) | <0.001h | 0.982h |
| Oocytes retrieved (OR)e | ||||||||||||||
| MeanIQR | 6 (5, 9) | 6 (4, 9) | 6 (5, 9) | 10 (7, 12) | 8 (7, 11) | 11 (8, 13) | 12 (8, 16) | 11 (7, 15) | 13 (11, 18) | 14 (10, 18) | 12 (9, 16) | 15 (11, 20) | <0.001g | <0.001g |
| According to target | <0.001h | <0.001h | ||||||||||||
| Below target (<7 oocytes), n (%) | 92 (60) | 45 (63) | 47 (57) | 32 (21) | 22 (26) | 10 (14) | 18 (12) | 17 (22) | 1 (1) | 6 (4) | 6 (8) | 0 (0) | ||
| Within target (7–15 oocytes), n (%) | 60 (39) | 26 (36) | 34 (41) | 109 (70) | 57 (66) | 52 (74) | 93 (60) | 48 (61) | 45 (58) | 83 (55) | 46 (61) | 37 (48) | ||
| Above target (>15 oocytes), n (%) | 2 (1) | 1 (1) | 1 (1) | 15 (10) | 7 (8) | 8 (11) | 45 (29) | 14 (18) | 31 (40) | 63 (41) | 23 (31) | 40 (52) | ||
| TQEsf | 0.6 (0.9) | 0.5 (0.9) | 0.7 (1.0) | 0.9 (1.3) | 1.0 (1.1) | 0.9 (1.5) | 1.1 (1.6) | 1.0 (1.4) | 1.2 (1.7) | 1.4 (2.1) | 1.5 (2.3) | 1.3 (2.0) | <0.001g | 0.830g |
| TQE/OR (%)f | 8.6 (14.9) | 8.4 (16.5) | 8.8 (13.5) | 9.5 (13.2) | 10.9 (13.5) | 7.8 (12.7) | 9.3 (11.9) | 9.9 (12.5) | 8.6 (11.3) | 10.2 (15.0) | 11.9 (17.3) | 8.4 (12.1) | 0.290g | 0.094g |
| Ongoing pregnancy, n (%) | 29 (19) | 9 (12) | 20 (24) | 52 (33) | 26 (30) | 26 (37) | 39 (25) | 26 (33) | 13 (17) | 41 (26) | 25 (33) | 16 (20) | 0.915h | 0.374h |
a<16.8 pmol/l.
b16.8–26.0 pmol/l.
c26.1–37.4 pmol/l.
d>37.4 pmol/l.
eMedian (IQR).
fMean (SD).
gANCOVA.
hLogistic regression.
Therefore, across the AMH quartiles, there were significant differences between the treatment groups in terms of dose adjustments on Day 6 (P = 0.034), mean gonadotrophin dose (P = 0.047) and total gonadotrophin dose (P = 0.025), duration of stimulation (P = 0.042) and ovarian response with respect to number of oocytes retrieved below/above the stimulation target (P < 0.001).
Serum AMH association with mid-follicular gonadotrophin-dose adjustments
Table III presents baseline characteristics, stimulation characteristics, embryo quality and pregnancy rates in relation to the gonadotrophin-dose adjustments on stimulation Day 6. The patients who increased the daily dose from 225 to 300 IU had a lower median serum AMH concentration prior to stimulation, received a higher total gonadotrophin dose and had slightly longer stimulation periods compared with the other patients. Despite this, cycle cancellation rate due to poor response or response below the target appeared more frequently in the group of patients with dose increases compared with the patients who continued on the starting dose. The patients who decreased the dose to 150 IU at stimulation day 6 appeared to have a higher median serum AMH concentration prior to stimulation and they consumed less gonadotrophin, had slightly shorter stimulation periods and a higher incidence of cancellations due to excessive response and of moderate/severe early OHSS compared with the patients with no dose adjustment.
Table III.
Baseline, stimulation and outcome variables in relation to gonadotrophin-dose adjustments on stimulation day 6.
| Variable | Dose adjustment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Increase |
No change |
Decrease |
|||||||
| All (n = 161) | HP-hMG (n = 90) | rFSH (n = 71) | All (n = 406) | HP-hMG (n = 200) | rFSH (n = 206) | All (n = 56) | HP-hMG (n = 24) | rFSH (n = 32) | |
| Serum AMH at the start of stimulation (pmol/l)a | 16.9 (11.8, 24.4) | 18.7 (13.8, 28.7) | 14.8 (10.3, 21.6) | 27.9 (18.9, 38.0) | 27.6 (19.6, 38.6) | 28.2 (18.5, 37.2) | 47.9 (34.2, 57.7) | 41.3 (27.5, 52.1) | 49.4 (36.6, 60.1) |
| Age (years)b | 31.9 (3.3) | 31.9 (3.1) | 31.8 (3.5) | 30.6 (3.2) | 30.4 (3.2) | 30.7 (3.3) | 29.4 (3.1) | 30.2 (3.3) | 28.9 (2.9) |
| AFCa | 9 (6, 12) | 9 (7, 12) | 9 (6, 11) | 10 (7, 14) | 10 (7, 15) | 10 (7, 14) | 15 (10, 21) | 13 (10, 19) | 16 (11, 24) |
| FSH at the start of stimulation (IU/l)a | 3.5 (2.8, 4.6) | 3.6 (2.8, 4.6) | 3.4 (2.8, 4.6) | 3.8 (3.0, 4.9) | 3.8 (3.0, 4.8) | 3.9 (3.0, 5.0) | 3.5 (2.9, 4.4) | 3.6 (2.6, 4.6) | 3.5 (3.1, 4.3) |
| Gonadotrophin dose on stimulation day 6 (IU) | 300 | 300 | 300 | 225 | 225 | 225 | 150 | 150 | 150 |
| Number of treatment daysb | 11.5 (2.0) | 11.7 (2.1) | 11.4 (2.0) | 10.1 (1.5) | 10.1 (1.6) | 10.0 (1.3) | 9.0 (1.6) | 9.3 (1.6) | 8.8 (1.5) |
| Total gonadotrophin dose (IU)b | 3164 (780) | 3212 (808) | 3102 (746) | 2267 (415) | 2282 (459) | 2252 (368) | 1714 (227) | 1744 (238) | 1692 (220) |
| Cycle cancelled | |||||||||
| Poor response, n (%) | 10 (6) | 8 (9) | 2 (3) | 6 (1) | 2 (1) | 4 (2) | 0 (0) | 0 (0) | 0 (0) |
| High response, n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (1) | 2 (1) | 3 (1) | 4 (7) | 1 (4) | 3 (9) |
| Early OHSS (moderate/severe), n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (1) | 3 (2) | 2 (1) | 3 (5) | 1 (4) | 2 (6) |
| Oocytes retrieved (OR)a | 8 (5, 11) | 8 (5, 10) | 8 (6, 12) | 11 (7, 15) | 10 (7, 13) | 12 (8, 16) | 15 (11, 20) | 12 (10, 15) | 17 (12, 20) |
| Below target, n (%) | 63 (39) | 38 (42) | 25 (35) | 82 (20) | 50 (25) | 32 (16) | 3 (5) | 2 (8) | 1 (3) |
| Within target, n (%) | 87 (54) | 45 (50) | 42 (59) | 232 (58) | 116 (59) | 116 (57) | 26 (46) | 16 (67) | 10 (31) |
| Above target, n (%) | 11 (7) | 7 (8) | 4 (6) | 87 (22) | 32 (16) | 55 (27) | 27 (48) | 6 (25) | 21 (66) |
| TQEsb | 0.6 (0.9) | 0.6 (0.8) | 0.6 (1.0) | 1.1 (1.7) | 1.2 (1.8) | 1.1 (1.6) | 1.3 (1.8) | 0.8 (1.2) | 1.6 (2.1) |
| TQE/OR (%)b | 7.6 (12.5) | 7.5 (11.6) | 7.6 (13.5) | 10.2 (14.4) | 12.0 (16.4) | 8.5 (11.9) | 8.3 (11.1) | 6.3 (8.8) | 9.9 (12.6) |
| Ongoing pregnancy, n (%) | 34 (21) | 20 (22) | 14 (20) | 109 (27) | 58 (29) | 51 (25) | 18 (32) | 8 (33) | 10 (31) |
aMedian (IQR).
bMean (SD).
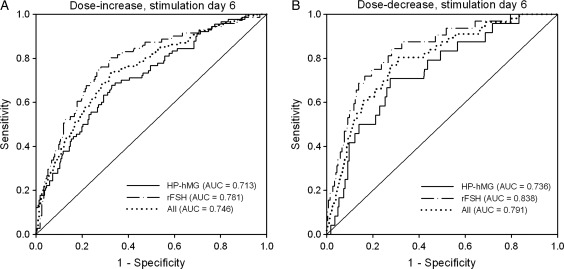
Figure 2 shows the ROC curve analyses performed to assess the predictive value of serum AMH at the start of the stimulation for the need for a gonadotrophin dose increase or dose decrease on stimulation day 6, respectively, for each treatment group and all patients. The predictive performance of the two models was high in both treatment groups (dose increase: 0.713 and 0.781 for HP-hMG and rFSH, respectively; dose decrease: 0.736 and 0.838 for HP-hMG and rFSH, respectively).
Figure 2.
ROC curve analysis showing the predictive value of serum AMH at the start of the stimulation for the need for a gonadotrophin-dose increase (A) or decrease (B) on stimulation day 6 in patients treated with HP-hMG or rFSH in the long GnRH agonist protocol. All patients had received starting doses of 225 IU/day for the first 5 days. The diagonal line is the reference line of no discrimination (AUC = 0.5). Cut-off values for increased dose were 23.0 pmol/l for HP-hMG (sens. 67.8%, spec. 67.1%) and 21.4 pmol/l for rFSH (sens. 74.6%, spec. 73.6%). The cut-off values for decreased dose were 32.4 pmol/l for HP-hMG (sens. 70.8%, spec. 72.6%) and 37.4 pmol/l for rFSH (sens. 75.0%, spec. 80.7%).
FF AMH at oocyte retrieval
Amongst the 731 patients who initiated COS, 582 patients had AMH measurements in fluid from one large follicle (≥17 mm) at oocyte retrieval: 290 patients treated with HP-hMG and 292 patients treated with rFSH. The median (IQR) concentration of FF AMH at oocyte retrieval was significantly higher in the HP-hMG group compared with the rFSH group [19.1 (14.2, 27.6) pmol/l versus 17.5 (12.0, 24.8) pmol/l, P = 0.007]. The AMH concentration in FF declined significantly with increased female age in both treatment groups. For the age categories 21–29, 30–34 and 35–37 years, the median (IQR) concentrations (pmol/l) in the HP-hMG group were 21.5 (15.0, 33.0; n = 100), 18.2 (14.9, 27.2; n = 151) and 14.8 (10.3, 23.0; n = 39), respectively and the corresponding values in the rFSH group were 19.6 (13.1, 26.9; n = 98), 16.5 (11.9, 24.3; n = 147) and 15.2 (9.8, 20.5; n = 47). As with for serum AMH, the coefficients for correlation between FF AMH and age were rather small in both treatment groups (HP-hMG group: r = −0.17, P = 0.004; rFSH group: r = −0.20, P < 0.001).
The AMH concentration in FF was significantly (P < 0.001) correlated with the AMH concentration in serum at the start of the stimulation, on Day 6 and on the last day of stimulation (HP-hMG group: r = 0.55, 0.59, and 0.64, respectively; rFSH group: 0.61, 0.59 and 0.63, respectively). All correlations between FF AMH and other FF endocrine variables (LH, hCG, inhibin A, estradiol, progesterone, androstenedione, total testosterone, SHBG, insulin, IGF-1, IGFBP-1, VEGF, cortisol and cortisone) were weak (r < 0.22) in both treatment groups, except for a negative correlation with FSH (HP-hMG group: r = −0.33, P < 0.001; rFSH group: r = −0.29, P < 0.001) and a positive correlation with inhibin B (HP-hMG group: r = 0.37, P < 0.001; rFSH group: r = 0.30, P < 0.001). The correlation between FF AMH and embryo quality was not estimated, since the AMH value of a specific follicle could not be linked to an individual oocyte/embryo.
Serum AMH association with outcome
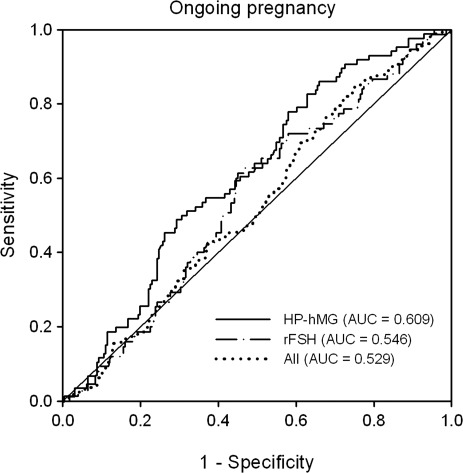
As shown in Table II, there was no significant linear association between serum AMH concentrations at the start of stimulation and ongoing pregnancy. ROC analyses were performed to assess the predictive value of serum AMH on stimulation day 1 for prediction of ongoing pregnancy. The predictive performance of serum AMH for ongoing pregnancy was low in both treatment groups (AUC = 0.609 and 0.546 in the HP-hMG and rFSH groups, respectively), as shown in Fig. 3.
Figure 3.
ROC curve analysis showing the predictive value of serum AMH at the start of the stimulation for ongoing pregnancy in patients treated with HP-hMG or rFSH in the long agonist protocol. The diagonal line is the reference line of no discrimination (AUC = 0.5).
Discussion
This study confirms that the serum AMH concentration during COS reflects the steady decline in the AMH-secreting follicle pool (Fanchin et al., 2003b, La Marca et al., 2004, Lee et al., 2010), as a significant gradual decrease of serum AMH was noticed in both the HP-hMG and rFSH treatment groups. The lower decline in serum AMH and the higher AMH concentration in FF at oocyte retrieval in the HP-hMG group compared with the rFSH group can be readily explained by the less pronounced follicle growth observed during the initial stimulation and the lower number of follicles/oocytes retrieved after stimulation with HP-hMG (Nyboe Andersen et al., 2006; Smitz et al., 2007).
The positive correlations between the serum concentrations of AMH and estradiol and androgens, measured during gonadotrophin stimulation, and with progesterone measured at oocyte retrieval may suggest that the AMH concentration after pituitary down-regulation reflects the capacity of a follicle pool to become steroidogenically active after stimulation and the luteinization potential of the selected cohort. The stronger correlation between serum AMH at the start of the stimulation and the serum estradiol concentration on stimulation day 6 in the rFSH group compared with the HP-hMG group may reflect the early estrogenization of a larger fraction of follicles recruited in the rFSH group (Nyboe Andersen et al., 2006; Smitz et al., 2007). Also, the apparent difference between the treatment groups regarding the correlation between serum AMH and the periovulatory progesterone concentration could imply a difference in the number of recruitable follicles achieving the pre-ovulatory stage as serum progesterone concentration at the end of the follicular phase has been shown to be related to FSH exposure and the number of growing follicles (Ubaldi et al., 1996; Fleming and Jenkins, 2010). In both treatments, there was a positive correlation between AMH in FF and inhibin B in FF supporting the view that the inhibin B concentration in FF reflects the quantity of immature granulosa cells in the small follicle pool (Groome et al., 1996; Welt et al., 1997).
The patients included in the present study were relatively young, in the narrow range between 21 and 37 years of age, and the observed inverse correlation between age and the serum concentration of AMH was weaker than previously described (van Rooij et al., 2005, Tremellen et al., 2005; Seifer et al., 2011; Nelson et al., 2011). Previous studies have shown that basal AMH and AFC assessed during the early follicular phase are highly correlated (van Rooij et al., 2002; de Vet et al., 2002; Fanchin et al., 2003a; Eldar-Geva et al., 2005), and both factors are considered to have a similar clinical value for the prediction of poor or excessive ovarian responses (Broer et al., 2009, 2010, 2011; La Marca et al., 2010). The present analysis verified the strong positive relationship between the number of oocytes retrieved and the serum AMH concentration prior to, during and at the end of the stimulation in both treatment groups. The finding that AMH was more strongly correlated with the number of oocytes retrieved than was AFC may be explained by the fact that the AFC data in the present study were derived from a large multicentre trial, while the studies included in the meta-analyses by Broer et al. (2009, 2011) were single-centre studies with potential less variation in the operator-dependent AFC assessments.
In line with the vast majority of published studies evaluating the value of AMH as a predictive marker in ART treatment (reviewed by Broer et al., 2010; La Marca et al., 2010), this study demonstrated that serum AMH is a predictive factor of oocyte quantity rather than quality, irrespective of the gonadotrophin preparation used. Hence, the ROC curve analyses indicated that the AMH concentration at the start of the stimulation was a good predictor of poor as well as excessive ovarian response in both treatment groups, while the number of TQEs (when accounting for the number of oocytes retrieved) and ongoing pregnancy rates were not different between patients with high and low basal AMH concentrations.
It has been suggested previously that the FF AMH concentration at oocyte retrieval, but not the serum AMH concentration, is positively associated with embryo implantation and ongoing pregnancy rates (Fanchin et al., 2007). However, the findings of the present study indicated a strong correlation between the FF AMH concentration and the serum AMH concentration on the last stimulation day which suggests that the FF AMH level reflects the circulating AMH level, i.e. the whole cohort of AMH-secreting antral follicles, and not the specific AMH production of the recruited large follicle in which AMH expression is considered to be undetectable (Weenen et al., 2004). This implies that embryo quality may not be directly related to the FF AMH level at oocyte retrieval. The lack of a significant association between serum AMH and pregnancy rate in the present study is in accordance with observations in several earlier studies (van Rooij et al., 2002; Peñarrubia et al., 2005; Fiçicioglu et al., 2006; Smeenk et al., 2007). So far, the attempts to identify cut-off levels for serum AMH that are able to distinguish between pregnancy and non-pregnancy have not been successful as reviewed by Broer et al. (2010). Only a few studies that were either limited by small numbers of patients (Eldar-Geva et al., 2005; Elgindy et al., 2008) or a retrospective observational design (Lekamge et al., 2007) have suggested that AMH can predict pregnancy outcome during IVF treatment. However, their findings may be explained, at least in part, by increased oocyte availability in patients with high AMH compared with patients with low AMH.
In the current study, the clinical consequences of a fixed starting dose for all patients, irrespective of the different serum AMH concentrations at the start of the stimulation, were readily observable as dose adjustments on stimulation day 6 were applied in approximately one-third of the study population. Since the ROC curve analysis showed that basal AMH was predictive of the need for a dose increase or decrease in both treatment groups, it may be hypothesized that if starting doses of gonadotrophins were set according to basal AMH concentrations, there may have been more patients within target of stimulation and less cancellations for poor or excessive response. A prospective study should explore whether starting gonadotrophin doses based on basal serum AMH values will optimize the ovarian response, reduce cycle cancellation rates and reduce the risks associated with excessive response.
In conclusion, the present study provides additional data to support the clinical value of AMH in COS cycles following the long GnRH agonist protocol. The study revealed a strong positive relationship between serum AMH and ovarian response, to HP-hMG as well as to rFSH, in terms of oocytes retrieved and the accompanying endocrine response. AMH is a good predictor of the need for gonadotrophin-dose adjustment on stimulation day 6 for patients with a fixed starting dose, but it does not predict embryo quality or ongoing pregnancy rates. AMH may guide the selection of patient-tailored gonadotrophin starting doses for a more optimal ovarian response, and, more importantly, for reducing the clinical risks associated with excessive response.
Authors' roles
E.A. was involved in data interpretation, manuscript drafting and critical discussion; J.S. was involved in study design, data interpretation, manuscript drafting and critical discussion; B.M.K. contributed to statistical analysis and critical discussion. J.S. contributed to assaying samples, data analysis and interpretation; J.-C.A. contributed to study design, data collection, manuscript drafting and critical discussion; all authors revised the manuscript and approved the final version.
Funding
The study was sponsored by Ferring Pharmaceuticals A/S, Copenhagen, Denmark. Funding to pay the Open Access publication charges for this article was provided by Ferring Pharmaceuticals A/S.
Conflict of interest
B.M.K. and J.-C.A. are employees of Ferring Pharmaceutical A/S.
Acknowledgement
The authors thank Göran Pettersson, PhD, Ferring Pharmaceuticals A/S, Copenhagen, Denmark for support with the preparation of this manuscript.
References
- Arce J-C, Nyboe Andersen A, Collins J. Resolving methodological and clinical issues in the design of efficacy trials in assisted reproductive technologies: a mini-review. Hum Reprod. 2005;20:1757–1771. doi: 10.1093/humrep/deh818. doi:10.1210/en.136.11.4951. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962. doi: 10.1210/endo.136.11.7588229. doi:10.1210/en.136.11.4951. [DOI] [PubMed] [Google Scholar]
- Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–714. doi: 10.1016/j.fertnstert.2007.12.013. doi:10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Broer SL, Mol B, Dólleman M, Fauser BC, Broekmans FJ. The role of anti-Müllerian hormone assessment in assisted reproductive technology outcome. Curr Opin Obstet Gynecol. 2010;22:193–201. doi: 10.1097/GCO.0b013e3283384911. doi:10.1097/GCO.0b013e3283384911. [DOI] [PubMed] [Google Scholar]
- Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17:46–54. doi: 10.1093/humupd/dmq034. doi:10.1093/humupd/dmq034. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. doi:10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. doi:10.1016/0092-8674(86)90783-X. [DOI] [PubMed] [Google Scholar]
- de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. doi:10.1016/S0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. doi:10.1210/en.140.12.5789. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. doi:10.1210/en.142.11.4891. [DOI] [PubMed] [Google Scholar]
- Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. doi:10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, Gal M, Zylber-Haran E, Margalioth EJ. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20:3178–3183. doi: 10.1093/humrep/dei203. doi:10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril. 2008;89:1670–1676. doi: 10.1016/j.fertnstert.2007.05.040. doi:10.1016/j.fertnstert.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003a;18:323–327. doi: 10.1093/humrep/deg042. doi:10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Schonäuer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003b;18:328–332. doi: 10.1093/humrep/deg043. doi:10.1093/humrep/deg043. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Müllerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–927. doi: 10.1093/humrep/deh688. doi:10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, Frydman R, Taieb J. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796–1802. doi: 10.1210/jc.2006-1053. doi:10.1210/jc.2006-1053. [DOI] [PubMed] [Google Scholar]
- Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antiMüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–596. doi: 10.1016/j.fertnstert.2005.09.019. doi:10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Fleming R, Jenkins J. The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod Biomed Online. 2010;21:446–449. doi: 10.1016/j.rbmo.2010.05.018. doi:10.1016/j.rbmo.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81:1401–1405. doi: 10.1210/jcem.81.4.8636341. doi:10.1210/jc.81.4.1401. [DOI] [PubMed] [Google Scholar]
- Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antiMüllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82:1323–1329. doi: 10.1016/j.fertnstert.2004.03.061. doi:10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, Lambalk C. Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008;90:737–743. doi: 10.1016/j.fertnstert.2007.07.1293. doi:10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, Volpe A. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19:2738–2741. doi: 10.1093/humrep/deh508. doi:10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–3107. doi: 10.1093/humrep/del291. doi:10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. doi:10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- Lee JR, Kim SH, Kim SM, Jee BC, Ku SY, Suh CS, Choi YM, Kim JG, Moon SY. Anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation and optimal timing of measurement for outcome prediction. Hum Reprod. 2010;25:2597–2604. doi: 10.1093/humrep/deq204. doi:10.1093/humrep/deq204. [DOI] [PubMed] [Google Scholar]
- Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Müllerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14:602–610. doi: 10.1016/s1472-6483(10)61053-x. doi:10.1016/S1472-6483(10)61053-X. [DOI] [PubMed] [Google Scholar]
- Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, de Jong FH, Fauser BJ, Laven JS. Anti-Müllerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality. Reprod Biomed Online. 2008;16:664–670. doi: 10.1016/s1472-6483(10)60480-4. doi:10.1016/S1472-6483(10)60480-4. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles-implications for individualization of therapy. Hum Reprod. 2007;22:2414–2421. doi: 10.1093/humrep/dem204. doi:10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, Mitchell P, Ambrose P, Fleming R. Anti-Müllerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–875. doi: 10.1093/humrep/den480. doi:10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Messow MC, McConnachie A, Wallace H, Kelsey T, Fleming R, Anderson RA, Leader B. External validation of nomogram for the decline in serum anti-Müllerian hormone in women: a population study of 15,834 infertility patients. Reprod Biomed Online. 2011;23:204–206. doi: 10.1016/j.rbmo.2011.05.006. doi:10.1016/j.rbmo.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Nyboe Andersen A, Devroey P, Arce J-C for the MERIT Group. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21:3217–3227. doi: 10.1093/humrep/del284. doi:10.1093/humrep/del284. [DOI] [PubMed] [Google Scholar]
- Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. doi:10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- Peñarrubia J, Fábregues F, Manau D, Creus M, Casals G, Casamitjana R, Carmona F, Vanrell JA, Balasch J. Basal and stimulation day 5 anti-Müllerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist-gonadotropin treatment. Hum Reprod. 2005;20:915–922. doi: 10.1093/humrep/deh718. doi:10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–471. doi: 10.1016/s0015-0282(01)03201-0. doi:10.1016/S0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Baker VL, Leader B. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–750. doi: 10.1016/j.fertnstert.2010.10.011. doi:10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, Keefe DL, Blazar AS. Müllerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159–163. doi: 10.1093/humrep/dei270. doi:10.1093/humrep/dei270. [DOI] [PubMed] [Google Scholar]
- Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Thomas CM, Braat DD. AntiMüllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223–226. doi: 10.1016/j.fertnstert.2006.06.019. doi:10.1016/j.fertnstert.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Smitz J, Nyboe Andersen A, Devroey P, Arce J-C for the MERIT Group. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22:676–687. doi: 10.1093/humrep/del445. doi:10.1093/humrep/del445. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20–24. doi: 10.1111/j.1479-828X.2005.00332.x. doi:10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- Ubaldi F, Camus M, Smitz J, Bennink HC, Van Steirteghem A, Devroey P. Premature luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist (GnRH-a) and recombinant follicle-stimulating hormone (FSH) and GnRH-a and urinary FSH. Fertil Steril. 1996;66:275–280. doi: 10.1016/s0015-0282(16)58453-2. [DOI] [PubMed] [Google Scholar]
- van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, Broekmans FJ. Comparison of inter- and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum Reprod. 2010;25:221–227. doi: 10.1093/humrep/dep366. doi:10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Themmen AP. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. doi:10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum antiMüllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. doi:10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. doi:10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Welt CK, Martin KA, Taylor AE, Lambert-Messerlian GM, Crowley WF, Jr, Smith JA, Schoenfeld DA, Hall JE. Frequency modulation of follicle-stimulating hormone (FSH) during the luteal-follicular transition: evidence for FSH control of inhibin B in normal women. J Clin Endocrinol Metab. 1997;82:2645–2652. doi: 10.1210/jcem.82.8.4138. doi:10.1210/jc.82.8.2645. [DOI] [PubMed] [Google Scholar]