Abstract
OBJECTIVE
We used a mixed-methods approach to explore the relationships between participants’ perceptions of family members’ diabetes self-care knowledge, family members’ diabetes-specific supportive and nonsupportive behaviors, and participants’ medication adherence and glycemic control (A1C).
RESEARCH DESIGN AND METHODS
Adults with type 2 diabetes participated in focus group sessions that discussed barriers and facilitators to diabetes management (n = 45) and/or completed surveys (n = 61) to collect demographic information, measures of diabetes medication adherence, perceptions of family members’ diabetes self-care knowledge, and perceptions of family members’ diabetes-specific supportive and nonsupportive behaviors. Most recent A1C was extracted from the medical record.
RESULTS
Perceiving family members were more knowledgeable about diabetes was associated with perceiving family members performed more diabetes-specific supportive behaviors, but was not associated with perceiving family members performed fewer nonsupportive behaviors. Perceiving family members performed more nonsupportive behaviors was associated with being less adherent to one’s diabetes medication regimen, and being less adherent was associated with worse glycemic control. In focus groups, participants discussed family member support and gave examples of family members who were informed about diabetes but performed sabotaging or nonsupportive behaviors.
CONCLUSIONS
Participant reports of family members’ nonsupportive behaviors were associated with being less adherent to one’s diabetes medication regimen. Participants emphasized the importance of instrumental help for diabetes self-care behaviors and reported that nonsupportive family behaviors sabotaged their efforts to perform these behaviors. Interventions should inform family members about diabetes and enhance their motivation and behavioral skills around not interfering with one's diabetes self-care efforts.
For adults with type 2 diabetes, the performance of diabetes self-care activities is associated with improved glycemic control and prevents diabetes-related complications, hospitalizations, and mortality (1). Most theories of health behavior change required for diabetes self-care performance include a social support component (2–4), and family members are considered a significant source of social support for adults with diabetes (5,6). Family members can have a positive and/or negative impact on the health of people with diabetes, interfere with or facilitate self-care activities (e.g., by buying groceries or refilling a prescription), and contribute to or buffer the deleterious effects of stress on glycemic control (7). Although family members can provide many kinds of social support (e.g., emotional, informational, and appraisal support), instrumental support (i.e., observable actions that make it possible or easier for an individual to perform healthy behaviors) has been most strongly associated with adherence to self-care behaviors across chronic diseases (8).
Even with correlational evidence supporting the importance of instrumental support, interventions rarely target family support as a means of promoting diabetes self-care behaviors among adults. Most diabetes intervention trials examine the effect of individual education on glycemic control, without engaging or educating family members or accounting for family member support as a process outcome (9). The few interventions for adults with diabetes that have included family members have been both largely inconsistent in their approach and ineffective in influencing health outcomes (10,11). For example, Kang et al. (12) tested an intervention that included individual family education sessions, group family education sessions, and weekly phone calls over 6 months. Participants in the family intervention reported an increase in family members’ supportive behaviors and a decrease in family members’ nonsupportive behaviors. Improvements in self-reported diabetes self-care behaviors, weight, and glycemic control were noted, although these observed changes were not significant (likely due to small sample size). In addition, Wing et al. (13) compared the efficacy of a weight loss intervention with spouses against an individual weight loss intervention and found no significant effect of the spousal intervention on exercise, caloric intake, weight loss, or glycemic control compared with the individual intervention. Gilliland et al. (14) conducted a three-arm intervention trial in Native American communities that included psychoeducational groups with adults with diabetes and their family members, one-on-one psychoeducational sessions without family members, and a control group. Inconsistent with predictions, the intervention groups demonstrated small increases in glycemic control relative to the control group. However, participants were not randomized to condition, and the study did not assess the interventions' effects on diabetes self-care behaviors. Thus, further work is needed to develop efficacious family-based interventions for adults with diabetes.
In our focus groups, adult participants with diabetes spontaneously discussed family member support when asked about their daily self-care regimens, underlining the influence of family support on diabetes self-care behaviors (15). On the basis of these in vivo findings, we used a mixed-methods approach with the same dataset to develop an understanding of the role of family support in the performance of self-care behaviors in general, and medication adherence specifically. Our objectives were to 1) explore the relationship between family members’ support and participants’ medication adherence and 2) expand the knowledge of what should be included in family-based interventions for adults with diabetes.
RESEARCH DESIGN AND METHODS
Participants and recruitment
Focus groups were conducted as a part of a larger project studying barriers and facilitators of diabetes medication adherence and the use of technology to manage diabetes and medication regimens. From June to December 2010, we recruited English-speaking adults diagnosed with type 2 diabetes who were prescribed glucose-lowering diabetes medications. Research assistants approached participants in clinic waiting rooms and responded to inquiries about the study from fliers or listserv announcements.
Of those eligible who consented to participate (N = 75), 61% (n = 45) attended a focus group session that included a discussion, survey, refreshments, and $40 compensation. All enrolled participants who did not attend a focus group were invited to complete the survey by phone and/or mail and received $20 compensation. Sixteen additional participants completed the survey, providing quantitative data for n = 61. The Vanderbilt University Medical Center Institutional Review Board approved all procedures prior to participant enrollment.
Data and procedure
Qualitative.
We conducted 11 focus groups with two to six participants, a trained facilitator (L.S.M. or C.Y.O.), and a trained note taker. Each focus group included an ∼60-min discussion and ∼20–30-min survey. Consistent with the parent study protocol, focus group questions pertained to barriers and facilitators of medication adherence, experiences with and attitudes toward using health information technology to manage diabetes and medication regimens, and ideas for leveraging technology to improve diabetes self-care. Focus group sessions were stratified by participants’ self-reported frequency of health information technology use to facilitate discussion specific to the parent study research questions (15). Thus, we did not have a priori questions about the role of family members in participants’ self-care behaviors. Rather, participants interjected this information into the larger discussion. All sessions were emergent, semistructured, and allowed the facilitator to adapt questions to participants’ experiences. Discussions were audiotaped and transcribed verbatim.
Quantitative.
A brief survey collected demographic information, including participants’ age, sex, race/ethnicity, education, household income, and marital status. Participants also reported perceptions of family members’ diabetes self-care knowledge and completed validated measures of perceptions of family members’ diabetes-specific supportive and nonsupportive behaviors and their own adherence to diabetes medications. Family members included any individuals the participant considered part of his/her family, as the survey did not specify a definition of “family.”
Family knowledge about diabetes self-care.
Perceptions of family members’ diabetes self-care knowledge was assessed by asking, “Generally, how much are your family members informed about what diabetes is and what it takes to manage it?” Responses were on a four-point scale, ranging from 1 (not at all) to 4 (a lot).
Family supportive and nonsupportive behaviors.
Perceptions of family members’ diabetes-specific supportive and nonsupportive behaviors were assessed with adapted subscales from the Diabetes Family Behavior Checklist (DFBC) (16,17). Since our sample included participants prescribed oral agents and/or insulin, participants could indicate “not applicable” for insulin-specific items. Therefore, the DFBC for participants prescribed insulin consisted of 16 items, whereas the DFBC for participants prescribed only oral agents consisted of 13 items. Items assessing supportive behaviors included questions such as, “How often do your family members exercise with you?” and “How often do your family members eat at the same time that you do?” Nonsupportive items included questions such as “How often do your family members criticize you for not exercising regularly?” and “How often do your family members argue with you about your diabetes self-care activities?” Responses ranged from 1 (never) to 5 (at least once a day), with higher scores indicating family members perform more supportive or nonsupportive behaviors, respectively. We averaged applicable items for each participant to create supportive and nonsupportive scaled scores. For participants who were prescribed insulin (n = 17), the internal consistency reliability (Cronbach α) of the 16-item DFBC was 0.82 for the supportive subscale and 0.74 for the nonsupportive subscale. For participants only on oral agents (n = 44), the internal consistency reliability of the 13-item DFBC (i.e., three insulin-specific items were removed) was 0.79 for the supportive subscale and 0.73 for the nonsupportive subscale.
Medication adherence.
Adherence to oral diabetes medications and insulin was assessed using the 12-item Adherence to Refills and Medication Scale (ARMS), which includes a four-item refill adherence subscale and an eight-item dose adherence subscale (18). We slightly modified each item to focus on diabetes medications (e.g., “How often do you forget to take your diabetes medicine or insulin?”). The ARMS is a reliable and valid instrument for assessing medication adherence (18) and has been shown to predict glycemic control (19). Response options are on a four-point scale, ranging from 1 (none of the time) to 4 (all of the time), and are summed to produce an overall adherence score ranging from 12 to 48, with higher scores representing worse medication adherence. In our sample, internal consistency reliability was 0.75.
Glycemic control.
Glycemic control was assessed by the most recent glycated hemoglobin (A1C) value in the medical record.
Analyses.
All statistical tests were performed using STATA version 11. Descriptive statistics characterized the sample. We conducted Shapiro-Wilk tests of normality and then used independent-sample t tests to explore group differences for normally distributed variables and Mann-Whitney U tests for nonnormally distributed variables. We previously examined demographic differences between focus group participants (n = 45) and nonparticipants (n = 16) and found no differences (15). We tested relationships between demographic, family, and outcome variables (i.e., medication adherence and glycemic control) using independent-sample t tests, Mann-Whitney U tests, or Spearman correlation coefficients (ρ) as appropriate.
Audiotapes were transcribed verbatim. We used NVivo 9 to code, analyze, and interpret the transcripts using elements of grounded theory (20). First, we identified all references to family members in the transcripts. We then conducted thematic analysis on participant comments about family members, excluding comments about a family history of diabetes. Identified major themes included 1) support from family members and 2) family members’ nonsupportive behaviors. We then used comparative analysis to categorize participant comments about family support as either instrumental, informational, emotional, or appraisal support. Comparative analyses of family members’ nonsupportive behaviors led to the development of two subthemes as follows: 1) sabotaging behaviors and 2) miscarried help.
Efforts to ensure quality.
To ensure the trustworthiness of our methodological approach, we participated in, recorded, and transcribed debriefing sessions after each focus group to discuss emerging themes. We also used methodological and analyst triangulation (21). Disagreements about the meaning of a participant comment or the type of support described were resolved through discussion and consensus. In the event a participant comment could not be clearly identified as supportive or nonsupportive, it was excluded from the analysis. Finally, family support emerged without facilitator inquiry in 11 focus groups with two different facilitators. Thus, the consistency of participant comments about family involvement across groups enhances the validity of our qualitative results (22).
RESULTS
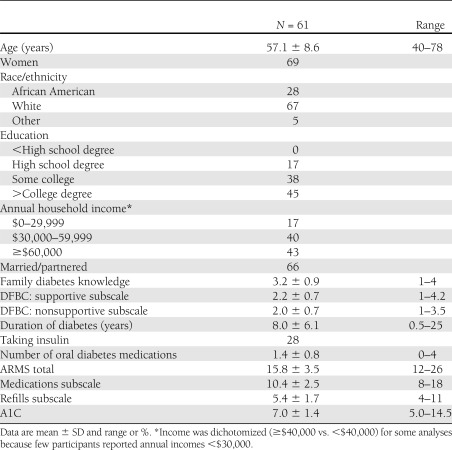
Table 1 presents the characteristics of participants who completed the survey with means (M) and SD or percentages. Separate sample characteristics for focus group-only participants have been previously reported (15). Results are reported as M and SD. On average, participants reported that their family members were “somewhat” informed about what diabetes is and what it takes to manage it. Both DFBC subscale distributions were nearly normal and had a slight positive skew. A1C scores ranged from 5.0 to 14.5, with an average A1C of 7.0 (SD = 1.4). Based on the American Diabetes Association definition (23), 34% of the sample had uncontrolled glycemia. Focus group participants (n = 45) also reported that their family members were “somewhat” informed about diabetes (M = 3.1, SD = 0.9). They reported similar frequencies of family member supportive behaviors (M = 2.2, SD = 0.7) and nonsupportive behaviors (M = 2.1, SD = 0.6). The average ARMS score for focus group participants was 16.3 (SD = 3.6) and the average A1C was 6.9 (SD = 1.1). There were no significant differences between focus group attendees and survey-only participants on our variables of interest.
Table 1.
Characteristics of participants
There were no sex differences in perceptions of family members’ diabetes self-care knowledge, diabetes-specific supportive behaviors, or nonsupportive behaviors. Advancing age was associated with reporting family members perform fewer nonsupportive behaviors (ρ = −0.30, P < 0.05). Participants reporting incomes ≥$40,000 were more likely than those with incomes <$40,000 to report that their family members perform more diabetes-specific supportive behaviors (U = 263.5, P < 0.05). However, more education was associated with reporting one’s family members were less knowledgeable about diabetes (ρ = −0.29, P < 0.05). Married/partnered participants were more likely than single, divorced, or widowed participants to report that their family members were more knowledgeable about diabetes (U = 567.5, P < 0.05), and were more likely to report that their family members perform more nonsupportive behaviors (U = 560.0, P < 0.05). Nonwhite participants had higher A1C values than white participants (U = 371.5, P < 0.05). No other demographic characteristics were associated with our variables of interest.
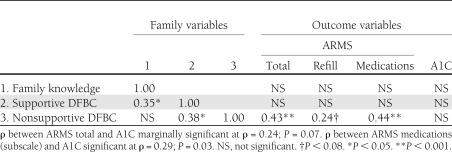
Spearman correlation coefficients between family variables, medication adherence, and glycemic control are presented in Table 2. Notably, perceiving one’s family members perform more nonsupportive behaviors was associated with reporting worse dose adherence to diabetes medications (ρ = 0.44, P < 0.001), which, in turn, was associated with higher A1C values (ρ = 0.29, P = 0.03). Perceiving one’s family members perform more nonsupportive behaviors was marginally associated with worse refill adherence (ρ = 0.24, P = 0.07). Thus, dose adherence drove the association between nonsupportive family behaviors and worse overall medication adherence (ρ = 0.43, P < 0.001). As noted in Table 2, perceiving one’s family members were more knowledgeable about diabetes was associated with perceiving one’s family members perform more diabetes-specific supportive behaviors, but was not associated with perceiving one's family members perform less nonsupportive behaviors. However, perceiving one's family members perform more supportive behaviors was also associated with perceiving one's family members perform more nonsupportive behaviors.
Table 2.
Spearman correlation coefficients between family variables and outcome variables
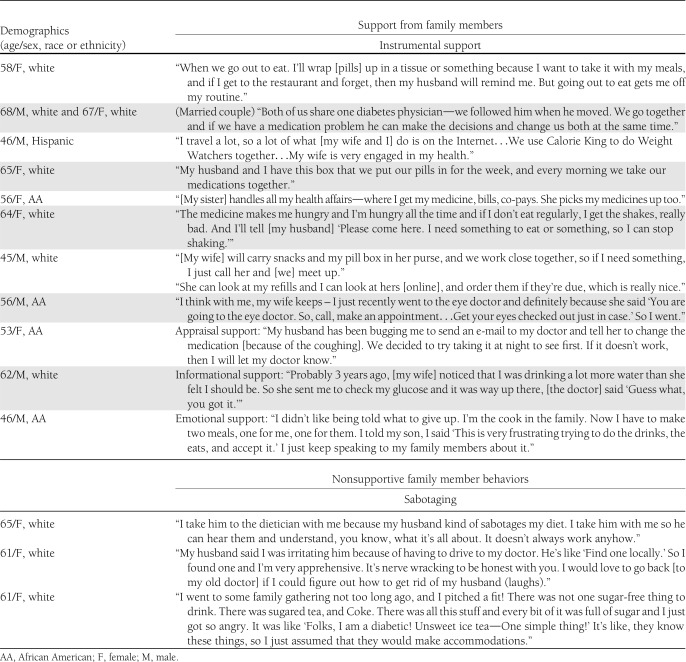
Focus group participants mentioned family involvement in all 11 focus groups (27 unique references). Illustrative statements are quoted verbatim below and in Table 3. Supportive family behaviors were mentioned in every focus group (18 unique references). Participants frequently discussed instrumental support (15 references) and rarely mentioned emotional (1 reference), informational (1 reference), or appraisal (1 reference) support. In light of the finding that perceiving one’s family members perform more nonsupportive behaviors was associated with less medication adherence, we share participants’ comments about their family members’ sabotaging behaviors (six references) and “miscarried help” (i.e., behaviors intended to be supportive that generate conflict, three references). Nonsupportive behaviors were discussed in four focus groups.
Table 3.
Select participant comments about family involvement in diabetes self-care regimen and participant demographics
Support from family members
Instrumental support was the most common form of family support discussed in the focus groups. Participants shared instrumental support they received from family members in areas such as diet, exercise, medication adherence, blood glucose monitoring, and managing doctors’ appointments. One participant shared that her husband was particularly supportive of her diabetes self-care activities. When asked by other group members how he supported her, she explained.
My husband does most of the grocery shopping, so he reads all the food labels for me and he will stand there in the aisles and read them—bless his heart—he is great like that. And he is into computers, so he will read stuff and he goes to the doctor and stuff with me whenever he can. (White female, age 56 years)
Several participants indicated that a family member’s awareness of their diabetes-specific needs made it easier to perform self-care behaviors. One participant shared how his wife always carried snacks and extra medication in her purse for him, and another shared how her sister understood her medication’s side effects and helped her manage them.
I have been taking my medicine in the morning, and I found that no matter how much breakfast I ate, about 2 hours after I took it, I was starting to feel not so good. Sunday it got really bad in church, and my sister saw [the effects]. She rushed off to the church’s kitchen and got some orange juice for me. (African American female, age 53 years)
Family members’ nonsupportive behaviors
Several participants expressed frustration with their family members’ nonsupportive behaviors, particularly in response to other participants’ comments about receiving instrumental support for performing self-care activities. Participants reported two types of nonsupportive behaviors: 1) sabotaging behaviors from family members who were well informed about diabetes but did not help the participant perform diabetes self-care behaviors and 2) miscarried helping behaviors, in which family members’ attempts to help with diabetes self-care produced conflict.
Sabotaging behaviors.
Some participants reported their family members (those with and without diabetes) were not motivated to make lifestyle changes or support their own diabetes self-care behaviors. These participants expressed frustration and voiced concerns that information about diabetes may be insufficient to motivate family members to perform supportive behaviors.
But, you know, I don’t think it’s enough [to research it with them]. The information can be out there and available, but how do you get somebody to care? (White female, age 65 years)
Participants shared stories when their family members had acted in nonsupportive ways despite knowing about dietary restrictions for people with diabetes, and sabotaging participants’ attempts to perform diabetes self-care behaviors.
What if you have a husband that’s a diabetic and he takes 1 pill a day and then he eats 24/7? As long as he’s awake, he is stuffing food in his face, and it is not good quality food. It’s doughnuts, chocolate cookies. And he’s a diabetic–never takes his blood sugar, and then he makes fun of me because I take my blood sugar 8 times a day and I have to be so careful! And he’s sticking these cookies in my face and he’s like “have a cookie” and I’m like “no thank you” ... he kind of sabotages me–but he has been a diabetic longer than I am. But he just–he doesn’t seem to care. He is just not interested. So, sometimes I don’t really have a support system. I am the support system. (White female, age 61 years)
One man described how his family members take him to restaurants where they know he will eat unhealthily.
If we are going to go to [restaurant name], they know I’m eating. That’s just it. I don’t think I can just sit there. If [my family] is going to give me some chicken fingers, they know I just can’t control it. Certain restaurants they want to go to—I’m getting it all—take some more insulin, pray over it, and go. (African American male, age 46 years)
Miscarried help.
In addition to family members performing sabotaging behaviors, some participants discussed instances of “miscarried help,” which produced conflict and interfered with self-care. For instance, a married couple shared their contrasting diabetes narratives as follows: the wife was insulin dependent, seldom followed a healthy diet, and had been hospitalized three times in the previous year, whereas the husband controlled his diabetes with diet and was largely without complications. The couple described experiencing conflict from the husband’s attempts to change the wife’s diet and the wife not appreciating her husband’s attempts to do so.
Another participant, whose spouse had diabetes but did not participate in the study, recounted how she had repeatedly threatened her husband in an attempt to get him to take his medicine and change his diet.
[I tell him] “Do you want to dance at our daughter’s wedding? You need feet to dance!” And I threaten him with—we have lots of animals, and I’m like—“Who’s going to take care of the animals? Not me! I’ll just open the gate and say bye!” (White female, age 61 years)
She reported these attempts had been unsuccessful in changing his behavior and joked about their marital conflict.
Miscarried help was also indicated in a relationship between a mother and her adult son who had recently been diagnosed with diabetes.
I don’t think [my son] got treated appropriately. I am still upset with his physician. And he wouldn’t listen to me either. He would just ignore everything I told him and go do his own searches [online], and it’s like “okay, I don’t know anything—I have been diabetic for 8 years—that’s okay, I guess I’m just your mom.” (White female, age 54 years)
CONCLUSIONS
We used a mixed-methods approach to examine the relationship between participant demographic characteristics, perceptions of family members’ diabetes self-care knowledge, perceptions of family members’ diabetes-specific supportive and nonsupportive behaviors, and participants’ diabetes medication adherence and glycemic control. Quantitative results indicated that perceiving family members had more diabetes self-care knowledge was associated with perceiving family members perform more diabetes-specific supportive behaviors. However, perceiving family members perform more nonsupportive behaviors was associated with reporting less adherence to diabetes medications, which, in turn, was associated with worse glycemic control. Interestingly, perceiving family members perform more diabetes-specific supportive behaviors was not associated with medication adherence or glycemic control, and family members’ supportive behaviors co-occurred with nonsupportive behaviors.
According to qualitative findings, participants think educating family members (i.e., providing them with information) about diabetes may not stop family members from performing nonsupportive, sabotaging behaviors. Participants reported feeling sabotaged by family members who are well informed about diabetes and its demands, but are unmotivated to make changes themselves or help the participant to make changes. In addition, we found that “miscarried help,” a concept introduced in the pediatric diabetes literature to explain interpersonal conflict that occurs when a caregiver attempts to supervise an adolescent’s self-care behaviors (24,25), may also be relevant to adults with diabetes. Miscarried helping behaviors are characterized by an intent to perform supportive behaviors that infringe upon an individual’s self-efficacy (24) and lead to relationship conflict about diabetes that has been associated with rebellion and poor health outcomes in adolescents with the condition (24,25). This construct is distinct from sabotaging behaviors, in which family members know that the individual should perform a healthy behavior, but encourage the individual to perform an unhealthy behavior. Moreover, overly solicitous behaviors are associated with lower self-reported diabetes self-efficacy and less physical activity, even when people with diabetes perceive these behaviors as helpful (26). Thus, family members who are too involved in diabetes management can create conflict and undermine an individual’s success at performing diabetes self-care activities. In our study, participants reported both receiving unappreciated help from family members and performing nagging or threatening behaviors to encourage self-care behaviors in their other family members who have diabetes. This evidence, although preliminary, presents an area for further research.
There are several limitations associated with this study. Participants were recruited from a single site and self-selected to attend a focus group and/or complete the survey, thereby limiting the generalizability of our results. In addition, the parent study was designed with different foci, so we did not prevent family members with a diabetes diagnosis from attending the same focus group. Three couples and one pair of sisters attended focus groups together, which may have increased discussion about family members' supportive behaviors or decreased discussion about family members’ nonsupportive behaviors. In qualitative research, saturation is reached when data collection no longer yields new results (27). The parent study achieved saturation regarding the use of health information technology to manage diabetes. We achieved saturation regarding the role of family members' supportive behaviors, but have likely only begun to understand the role of family members' nonsupportive behaviors and miscarried help.
We used quantitative and qualitative methods in concert to understand a phenomenon from different perspectives. Consequently, the statistical power of our quantitative results is limited by our qualitative approach to data collection and sampling, and we were unable to control for variables that might confound the relationships of interest. Further, our study presents participants’ perceptions of family members’ knowledge and supportive/nonsupportive behaviors, which may not adequately reflect actual family members’ knowledge and behaviors. Finally, our cross-sectional design limits our ability to discern causal relationships, and further evidence is necessary to make strong conclusions about the role of family members’ diabetes self-care knowledge and supportive/nonsupportive behaviors, and one’s medication adherence and glycemic control.
Our results are consistent with other studies reporting that social support directly affects adults’ performance of diabetes self-care behaviors and indirectly affects their glycemic control (4,28). Although other studies have reported that nonsupportive behaviors hinder the ability of adults with diabetes to perform certain self-care activities (16,17,29), to our knowledge, this is the first examination of the relationship between family members' supportive/nonsupportive behaviors and diabetes medication adherence. Our mixed-methods approach provides a nuanced understanding of the role of family support in diabetes self-care from the perspectives of adults with diabetes. It is notable that family support was not an a priori focus of the parent study, and participants discussed family members' behaviors spontaneously and frequently, thus underlining its importance in diabetes management.
Our findings suggest a new direction for future work to develop effective family-based interventions for adults with diabetes. Future qualitative research should identify all relevant nonsupportive family behaviors and understand the role of miscarried helping among adults with diabetes. Additional quantitative research (e.g., prospective studies) is needed to understand the causal relationships between family members’ diabetes self-care knowledge and supportive/nonsupportive behaviors, and an individual’s diabetes self-care behaviors and health outcomes. That work should explore differences in the perceived helpfulness of family members' behaviors and differences in perceived and desired family support based on age, sex, time since diagnosis, race/ethnicity, socioeconomic status, and self-efficacy. Such research may facilitate the development of interventions for adults with diabetes that focus on reducing the frequency of family members’ communications or actions that interfere with the performance of one's diabetes self-care behaviors. Providers should discuss with family members the influence of instrumental support and nonsupportive behaviors on a patient’s self-care and health outcomes and help the patient develop strategies to address nonsupportive family member behaviors.
Acknowledgments
This research was funded with support from the Vanderbilt University Diabetes Research and Training Center Pilot and Feasibility grant (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] Grant P60-DK-020593) and a Career Development award (NIDDK K01-DK-087894-01A1 to C.Y.O.).
No potential conflicts of interest relevant to this article were reported.
L.S.M. facilitated focus group discussions, collected and managed data, conducted the analyses, and wrote the manuscript. C.Y.O. designed the parent study, facilitated focus group discussions, collected and managed data, conducted the analyses, contributed to the development of the INTRODUCTION and CONCLUSIONS, and reviewed and edited the manuscript. C.Y.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to thank the participants for their contributions to this work.
References
- 1.Sklyer JS, Bergenstal R, Bonow RO, et al. ; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation 2009;119:351–357 [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero L, Prochaska JO. Readiness for change: application of the transtheoretical model to diabetes. Diabetes Spectrum. 1993;6:22–60 [Google Scholar]
- 3.Tillotson LM, Smith MS. Locus of control, social support, and adherence to the diabetes regimen. Diabetes Educ 1996;22:133–139 [DOI] [PubMed] [Google Scholar]
- 4.Osborn CY, Egede LE. Validation of an information-motivation-behavioral skills model of diabetes self-care (IMB-DSC). Patient Educ Couns 2010;79:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang TS, Brown MB, Funnell MM, Anderson RM. Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. Diabetes Educ 2008;34:266–276 [DOI] [PubMed] [Google Scholar]
- 6.Fisher L, Chesla CA, Bartz RJ, et al. The family and type 2 diabetes: a framework for intervention. Diabetes Educ 1998;24:599–607 [DOI] [PubMed] [Google Scholar]
- 7.Fisher L, Chesla CA, Skaff MM, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care 2000;23:267–272 [DOI] [PubMed] [Google Scholar]
- 8.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol 2004;23:207–218 [DOI] [PubMed] [Google Scholar]
- 9.Norris SL, Engelgau MM, Narayan KMV. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561–587 [DOI] [PubMed] [Google Scholar]
- 10.van Dam HA, van der Horst FG, Knoops L, Ryckman RM, Crebolder HFJM, van den Borne BHW. Social support in diabetes: a systematic review of controlled intervention studies. Patient Educ Couns 2005;59:1–12 [DOI] [PubMed] [Google Scholar]
- 11.Armour TA, Norris SL, Jack LJ, Jr, Zhang X, Fisher L. The effectiveness of family interventions in people with diabetes mellitus: a systematic review. Diabet Med 2005;22:1295–1305 [DOI] [PubMed] [Google Scholar]
- 12.Kang C-M, Chang S-C, Chen P-L, et al. Comparison of family partnership intervention care vs. conventional care in adult patients with poorly controlled type 2 diabetes in a community hospital: a randomized controlled trial. Int J Nurs Stud 2010;47:1363–1373 [DOI] [PubMed] [Google Scholar]
- 13.Wing RR, Marcus MD, Epstein LH, Jawad AA. A “family-based” approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156–162 [DOI] [PubMed] [Google Scholar]
- 14.Gilliland SS, Azen SP, Perez GE, Carter JS. Strong in body and spirit: lifestyle intervention for Native American adults with diabetes in New Mexico. Diabetes Care 2002;25:78–83 [DOI] [PubMed] [Google Scholar]
- 15.Mayberry LS, Kripalani S, Rothman RL, Osborn CY. Bridging the digital divide in diabetes: family support and implications for health literacy. Diabetes Technol Ther 2011;13:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow RE, Toobert DJ. Social environment and regimen adherence among type II diabetic patients. Diabetes Care 1988;11:377–386 [DOI] [PubMed] [Google Scholar]
- 17.Schafer LC, Kevin MS, Mccaul D, Glasgow RE. Supportive and non-supportive family behaviors: relationships to adherence metabolic control in persons with type 1 diabetes. Diabetes Care 1986;9:225–234 [DOI] [PubMed] [Google Scholar]
- 18.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health 2009;12:118–123 [DOI] [PubMed] [Google Scholar]
- 19.Osborn CY, Quintero CC, Kripalani S, et al. Racial disparities in diabetes: health literacy, numeracy, or something else? Presented at the 71st Scientific Sessions of the American Diabetes Association, 24–28 June 2011, at the San Diego Convention Center, San Diego, CA [Google Scholar]
- 20.Strauss A, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 2nd ed. Thousand Oaks, CA, Sage Publications, 1998 [Google Scholar]
- 21.Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res 1999;34:1189–1208 [PMC free article] [PubMed] [Google Scholar]
- 22.Lincoln YS, Guba EG. Naturalistic Inquiry. Newbury Park, CA, Sage Publications, 1985 [Google Scholar]
- 23.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris MA. The family's involvement in diabetes care and the problem of ‘miscarried helping’. Business Briefing: European Endocrine Review 2006;Reference Section(January):1–3 [Google Scholar]
- 25.Anderson BJ, Coyne JC. “Miscarried helping” in families of children and adolescents with chronic diseases. In Advances in Child Health Psychology Johnson JH, Johnson SB, Eds. Gainesville, FL, University of Florida Press, 1991, p. 167–177 [Google Scholar]
- 26.Lyons RR, Sullivan MJL, Ritvo PG, Coyne JC. Relationships in Chronic Illness and Disability. Thousand Oaks, CA, Sage Publications, 1995 [Google Scholar]
- 27.Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. 4th ed. Thousand Oaks, CA, Sage Publications, 2009 [Google Scholar]
- 28.Osborn CY, Amico KR, Fisher WA, Egede LE, Fisher JD. An information-motivation-behavioral skills analysis of diet and exercise behavior in Puerto Ricans with diabetes. J Health Psychol 2010;15:1201–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen LK, Parchman ML, Shepherd MD. Family support and diet barriers among older Hispanic adults with type 2 diabetes. Fam Med 2004;36:423–430 [PubMed] [Google Scholar]