Abstract
OBJECTIVE
To determine tissue factor procoagulant activity (TF-PCA) in patients with type 1 diabetes and to examine effects of hyperglycemia and hyperglycemia plus hyperinsulinemia on TF-PCA.
RESEARCH DESIGN AND METHODS
We have determined circulating TF-PCA and other coagulation factors under basal (hyperglycemic) conditions, after acute correction of hyperglycemia, in response to 24 h of selective hyperglycemia, and in response to 24 h of hyperglycemia plus hyperinsulinemia in nine type 1 diabetic patients and in seven nondiabetic control subjects.
RESULTS
As shown previously in patients with type 2 diabetes, basal TF-PCA and plasma coagulation factor VIIa (FVIIa) were higher in patients with type 1 diabetes than in nondiabetic control subjects. However, in contrast with type 2 diabetes, normalizing glucose did not decrease the elevated TF-PCA levels, and raising glucose or glucose plus insulin levels did not increase TF-PCA.
CONCLUSIONS
Patients with type 1 diabetes have elevated circulating TF-PCA and FVIIa levels and are in a procoagulant state that may predispose them to acute cardiovascular events. The mechanisms regulating TF-PCA in patients with type 1 and type 2 diabetes are different and should be further explored.
Type 1 diabetes mellitus (T1DM) is associated with increased risk for atherosclerotic vascular disease (1). There are several well-recognized factors responsible for this enhanced risk, including hypertension, atherogenic dyslipidemia, smoking, and renal disease. Additional putative risk factors include the alterations in platelet function, plasma coagulation factors, and the fibrinolytic system that indicates a prothrombotic state (2–4). Thrombosis is a key factor in acute events associated with atherosclerotic vascular disease, including myocardial infarction, stroke, and peripheral arterial disease. Tissue factor (TF) is the primary physiological initiator of blood coagulation and thrombosis (5,6). The original concept that TF was present only in the adventitia of blood vessels and in atherosclerotic plaques, and initiates blood coagulation only when vessel walls were injured or plaques fissured (7), has recently been broadened by the demonstration that, in addition, there is a circulating pool of TF in blood that is thrombogenic (5,6,8–10).
We and others have previously shown that patients with type 2 diabetes mellitus (T2DM) have elevated levels of circulating TF procoagulant activity (TF-PCA) and are in a procoagulant state (11,12). Further, we have reported that raising blood insulin levels, and especially raising blood glucose and insulin levels together to levels frequently seen in diabetic patients, increased TF-PCA and thrombin generation in patients with T2DM and in nondiabetic volunteers (11,13). These findings support the notion that hyperglycemia and hyperinsulinemia are likely to contribute to the procoagulant state in patients with T2DM and to their predisposition for acute cardiovascular events. On the other hand, there is currently no information on circulating levels of TF-PCA and TF responses to hyperglycemia and hyperinsulinemia in patients with T1DM who are at comparable, if not greater, risk for cardiovascular events than patients with T2DM (4). Further, whether selective hyperglycemia (i.e., hyperglycemia without hyperinsulinemia) affects TF-PCA is not known. The reason is that to produce selective hyperglycemia in nondiabetic patients or in T2DM patients, somatostatin needs to be infused together with glucose to prevent hyperglycemia-induced hyperinsulinemia. Somatostatin, however, was found to have its own effects on TF-PCA (14). In contrast, in C-peptide–negative patients with T1DM, selective hyperglycemia can be produced without somatostatin. The objectives of this study, therefore, were to investigate in patients with T1DM levels of TF-PCA and other blood coagulation factors under basal (overnight fasted) conditions and in response to 24 h of selective hyperglycemia or combined hyperglycemia plus hyperinsulinemia.
RESEARCH DESIGN AND METHODS
Study subjects
Nine patients with T1DM and seven nondiabetic healthy control subjects were studied at the Clinical Research Center of Temple University Hospital (Table 1). In eight of the nine T1DM patients, basal C-peptide levels were very low (0.25 ± 0.09 ng/mL) and did not respond to intravenous glucose. One patient had a subnormal response to glucose (from 0.93 to 1.93 ng/mL). Six of the nine patients were taking antihypertensive medications, three of the nine were treated for hyperlipidemia, and three patients took aspirin. None had clinical signs of renal insufficiency and proliferative retinopathy. One patient had lower limb sensory neuropathy. Six patients with T1DM were on a basal/bolus insulin regimen consisting of insulin glargine in doses ranging from 15 to 70 units taken at night. Three patients took a NovoLog 70/30 mix (insulin asparte-protamine/insulin asparte) twice daily in doses ranging from 45 to 50 units. The nondiabetic control subjects were healthy, had no family history of diabetes, and did not take any medications. Informed written consent was obtained from all patients after explanation of the nature, purpose, and potential risks of the studies. The study protocol was approved by the institutional review board of Temple University Hospital.
Table 1.
Study subjects
Study design
Determination of TF-PCA and markers of blood coagulation in the basal state.
Study participants came to the Clinical Research Center (CRC) after an overnight fast. They had their last meal and insulin injection at ∼8:00 p.m. At ∼8:00 a.m. the next day, blood was collected for determination of circulating TF-PCA, plasma coagulation markers, and glucose and insulin concentrations.
On different days the following studies were performed:
Acute correction of hyperglycemia in patients with T1DM followed by selective hyperglycemia for 24 h. Seven patients with T1DM were admitted to the CRC. During the night, their blood glucose concentrations were normalized by intravenous infusion of regular insulin. The following morning, blood glucose levels were raised to ∼300 mg/dL (∼16.7 mmol/L) by intravenous infusion 20% glucose and maintained at this level for 24 h. The purpose of these studies was a) to examine the effects of acutely lowering and b) of acutely raising blood glucose levels on TF-PCA and other coagulation factors. Blood was collected at 6 h intervals for measuring of TF-PCA and blood coagulation factors, at 30 min intervals for determination of glucose, and at 2 h intervals for determination of insulin and C-peptide levels.
Combined elevation of glucose and insulin (high glucose/high insulin clamps) for 24 h. The purpose of these studies was to examine effects of raising both blood glucose and insulin levels on TF-PCA and other coagulation factors. A 20% glucose solution was infused intravenous at variable rates, which were adjusted to maintain plasma glucose at approximately 200–300 mg/dL (∼11–16 mmol/L). In diabetic patients, insulin was infused at a rate of 1 mU/kg min. In nondiabetic control subjects, insulin levels rose in response to hyperglycemia. Blood glucose concentrations and electrolyte and fluid balances were monitored as described above. Hyperglycemic-hyperinsulinemic clamps were continued for 8 h in five patients and for 24 h in three patients. Plasma electrolytes were monitored every 6 h, body weight every 12 h, and fluid balances every 6 h. Potassium (20 mg) and magnesium (400 mg) were administered orally every 12 h.
Assays
Blood samples were collected from antecubital veins without tourniquet-induced venostasis at 0, 6, 12, 18, and 24 h. Plasma glucose was measured with a glucose analyzer using the glucose oxidase method, serum insulin by radioimmunoassay (RIA) using an antiserum with minimal (<0.2%) cross-reactivity with proinsulin (Millipore Corp., Billerica, MA). Adiponectin was measured by RIA (Millipore Corp.) with an antiserum that detects total adiponectin. Serum triglycerides were measured enzymatically. Electrolytes were measured at the Temple University Hospital Chemistry Laboratory. A1C was measured with a Centers for Disease Control and Prevention–approved automated point-of-care analyzer (DCA2000; Bayer Corp., Tarrytown, NY).
TF-PCA was measured in whole-blood cell lysates with a two-stage clotting assay using recombinant FVIIa (American Diagnostica), Factor X (Enzyme Research Laboratories, South Bend, IN), and normal human plasma–containing phospholipids vesicles, as described previously (9,13). This assay measures cell-bound and microparticle-associated TF in lysed membranes obtained from whole blood.
Plasma FVIIa, FVIIc, and FVIIIc activities were measured with clotting assays as described (13). Plasma fibrinogen levels were measured by a one-stage clotting assay based on the method described by Clauss (15) using bovine thrombin and human fibrinogen reference plasma (Fisher Diagnostics, Middletown, VA). Thrombin generation was assessed by determination of thrombin-antithrombin (TAT) complexes in plasma using ELISA kits (Enzygnost; Dade Behring, Marburg, Germany). Plasma-soluble vascular cell adhesion molecule-1 (sVCAM-1) was measured by an ELISA (Gen-Probe Diaclone, Besançon, France).
Plasminogen activator inhibitor-1 (PAI-1) antigen was measured by an ELISA (American Diagnostica, Stamford, CT). For measurement of microparticles, one aliquot (1 mL) of citrated plasma samples was centrifuged at 13,000g for 2 min to remove any residual cells. The supernatant was carefully removed and placed in a 1.5-mL microcentrifuge tube and stored at −70°C. Procoagulant activity of microparticles in plasma was determined by a functional assay based on its annexin V–binding property with a commercially available kit (Zymuphen; HYPHEN BioMed, Neuville-sur-Oise, France).
Statistical analysis
A one-way ANOVA was used to test for significant differences between studies with Student-Newman-Keuls post hoc analysis. If data were not normally distributed, the Kruskal-Wallis one-way ANOVA with Dunn post hoc analysis was used. To test for differences across time, a one-way repeated-measures ANOVA with Student-Newman-Keuls post hoc analysis was used. If data were not normally distributed, the Friedman repeated-measures ANOVA on ranks with Student-Newman-Keuls post hoc analysis was used. Statistical differences between Day 1 (baseline) and Day 2 (24 h) were determined with a paired t test and, if not normally distributed, with the Wilcoxon signed rank test. Statistical analyses were performed using SigmaStat for Windows (version 2.0; SPSS, Chicago, IL). Statistical significance was defined as P < 0.05. All results are presented as means ± SE.
RESULTS
Blood glucose, TF-PCA, and FVIIa are elevated in T1DM
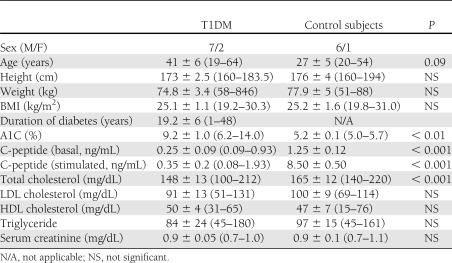
Basal blood glucose was 191 ± 39 mg/dL (10.6 ± 2.2 mmol/L) in nine patients with T1DM and 91 ± 4 mg/dL (5.1 ± 0.2 mmol/L) in seven nondiabetic control subjects (P < 0.04) (Fig. 1). With one exception, basal circulating TF-PCA levels were higher in T1DM patients than in control subjects (64.7 ± 6.0 vs. 24.6 ± 1.2 units/mL, P < 0.001) and were similarly elevated in patients who were in good (A1C ∼6.2%), fair (A1C ∼8%), or poor (A1C 14%) glycemic control. Mean basal circulating FVIIa concentrations were 2.7 times higher in T1DM patients than in control subjects (104 ± 24 vs. 38 ± 8 mU/mL, P < 0.03), and PAI-1 levels tended to be lower (45.3 ± 5.9 vs. 28.4 ± 14.9 ng/mL, P = 0.13). Plasma levels of FVIIc, FVIII, TAT, or VCAM were not significantly different in patients with T1DM and control subjects. Plasma adiponectin levels, however, were elevated in patients with T1DM compared with control subjects (12.4 ± 1.9 vs. 6.7 ± 2.0 μg/mL, P < 0.05).
Figure 1.
A: Open columns: Postabsorptive plasma (basal) glucose, circulating TF-PCA, and plasma FVIIa levels in patients with T1DM and nondiabetic control subjects (NC). Shown are means ± SE. HG, Patients with hyperglycemia. NG, Patients after rapid normalization of glucose levels. Crosshatched columns: Glucose, TF-PCA, and FVIIa levels in eight T1DM patients after the glucose levels were normalized overnight. B: Individual TF-PCA and A1C levels in nine patients with T1DM and seven nondiabetic control subjects.
Effects of acute normalization of plasma glucose
To explore effects of acutely normalizing plasma glucose concentrations, plasma glucose was lowered overnight in eight patients with T1DM to 94 ± 9 mg/dL (5.2 ± 0.5 mmol/L) over a 6.7 ± 2.7-h period (range 3–15 h) (T1DM, NG in Fig. 1) (Fig. 1, crosshatched columns). The acute normalization of blood glucose concentrations did not change TF-PCA or FVIIa, both of which remained elevated (71.9 ± 5.4 units/mL and 104.9 ± 17 mU/mL, respectively). There were also no changes in plasma concentrations of FVIIc and FVIII (0.96 ± 0.08 and 2.25 ± 0.4 units/mL, respectively).
Effects of raising glucose
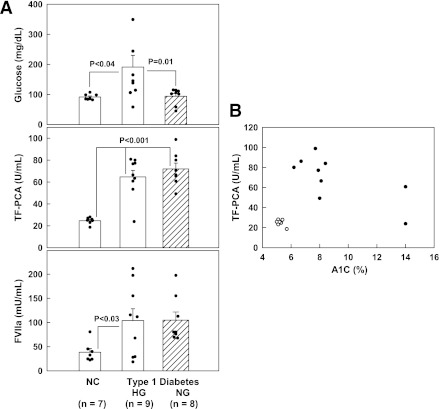
To explore the effects of acutely raising plasma glucose in diabetic patients, glucose was raised from 103 ± 8 to ∼300 mg/dL (from 5.7 ± 0.4 to ∼16.7 mmol/L) by infusion of 20% dextrose and maintained at this level for 24 h (Fig. 2, left panels). Insulin concentrations were kept at basal concentrations of between 6 and 13 μU/mL (35 and 75 pmol/L) by intravenous infusion of small amounts of regular insulin. TF-PCA decreased from 73 ± 6 units/mL at 0 h to 54 ± 7 units/mL at 24 h (P < 0.02). There were no significant changes in plasma concentrations of FVIIa (112 vs. 115 units/mL), FVIIc (0.95 vs. 0.93 units/mL), FVIII (2.4 vs. 2.1 units/mL), fibrinogen (363 vs. 357 ng/mL), VCAM (543 vs. 549 ng/mL), and TAT (12 vs. 12 μg/L). Basal microparticle (MP) levels were the same in diabetic and nondiabetic patients (4.2 ± 1.4 vs. 4.1 ± 1.1 nmol/L). Hyperglycemia had no effect on MP levels in nondiabetic patients, but it increased MP levels in diabetic patients (from 4.1 ± 1.1 to 15.3 ± 5.2 nmol/L, P < 0.01).
Figure 2.
Left columns: Plasma glucose and serum insulin concentrations, circulating TF-PCA, and FVIIa activity before and during 24 h of selective hyperglycemia in seven patients with T1DM. Shown are means ± SE. Statistical analysis: *P < 0.02 compared with 0 h. Right columns: Plasma glucose, serum insulin, circulating TF-PCA, and FVIIa levels before and during 10 h of combined hyperglycemia plus hyperinsulinemia in eight patients with T1DM and in seven nondiabetic control subjects (open circles). Shown are means ± SE. Statistical analysis: TF-PCA *P < 0.01 compared with baseline. FVIIa: *P < 0.05; **P < 0.01 compared with baseline.
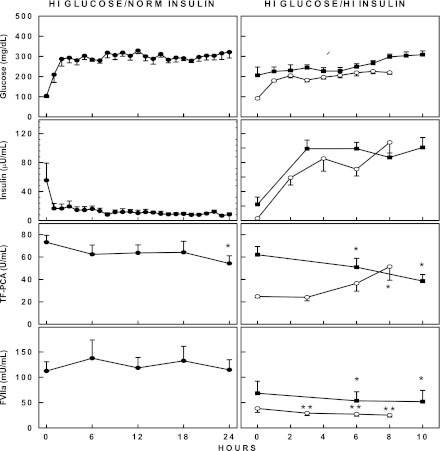
Effects of raising both glucose and insulin
In previous studies, raising glucose and insulin together produced the highest levels of TF-PCA in nondiabetic patients and in patients with T2DM (11,13). We therefore raised glucose to ∼250 mg/dL (∼14 mmol/L) and insulin to ∼100 μU/mL (∼600 pmol/L) for 10 h in eight patients with T1DM. For comparison and as a positive control, seven nondiabetic patients were studied for 8 h under similar hyperglycemic-hyperinsulinemic conditions. In patients with T1DM, TF-PCA declined from 62 ± 7 at 0 h to 39 ± 6 units/mL (P < 0.001) at 10 h. FVIIa declined from 69 to 52 units/mL (P < 0.05). In three patients, hyperglycemic-hyperinsulinemic clamps were extended to 24-h studies. In these three patients, TF-PCA declined from 55 ± 16 to 25 ± 3 units/mL at 24 h (not significant). FVIIc, FVIII, TAT, PAI-1, and VCAM did not change significantly. By comparison, TF-PCA in the nondiabetic control subjects increased from 25 ± 1 units/mL at 0 h to 51 ± 12 units/mL at 8 h (P < 0.05), and FVIIa decreased (from 38 to 25 units/mL, P < 0.002).
At the start of the clamps, basal plasma adiponectin levels were higher in the diabetic patients than in the nondiabetic control subjects (10.2 ± 2.1 vs. 5.9 ± 0.6 μg/mL, P < 0.03). Ten hours of hyperglycemic-hyperinsulinemia had no effect on adiponectin levels in diabetic patients (10.2 ± vs. 10.3 μg/mL) or in nondiabetic control subjects (5.9 vs. 5.5 μg/mL).
Similar to MP changes during hyperglycemia, MP levels rose from 4.8 ± 1.6 to 11.4 ± 3.1 nmol/L in response to hyperglycemic-hyperinsulinemia in diabetic patients.
CONCLUSIONS
An important finding in this study was that basal circulating TF-PCA and FVIIa levels in a small group of patients with T1DM were higher than those in nondiabetic control subjects (see Table 1). Most of our diabetic patients took medications, including aspirin and statins, which have been reported to decrease circulating TF (10,16,17). TF-PCA in these patients may therefore have been even higher without these medications. We and others have previously reported similarly elevated TF-PCA levels in patients with T2DM (11,12). There are reasons to believe that elevated TF-PCA levels in diabetic patients are clinically relevant. For instance, TF has been associated with increased blood thrombogenicity in patients with cardiovascular disease (18), sickle cell disease (9), hyperlipidemia (12), antiphospholipid antibody syndrome (19), and disseminated intravascular coagulation (20). Moreover, in patients undergoing angioplasty or stent implantation, elevated whole-blood TF-PCA present before the procedure predicted restenosis (16). In addition, the patients in this study had elevated plasma FVIIa levels, which is also associated with increased risk for acute cardiovascular events (18,21).
A second interesting finding was that TF-PCA responses to blood glucose normalization and to raising glucose alone or glucose together with insulin were distinctly different in patients with T1DM compared with patients with T2DM and nondiabetic patients. In patients with T1DM, the combination of hyperinsulinemia and hyperglycemia decreased TF-PCA levels, whereas, as shown previously, it strongly increased TF-PCA levels in patients with T2DM and in nondiabetic control subjects (11,13). In addition, 24 h of selective hyperinsulinemia (high insulin, normal glucose), which increased TF-PCA in nondiabetic patients and in patients with T2DM (11,13), was similarly ineffective in three patients with T1DM (data not shown). Thus, our studies indicated that the regulation of circulating TF-PCA levels in patients with T1DM and T2DM was different. We do not, at this time, understand the reason for the difference. Our previous results in patients with T2DM had suggested that their elevated TF-PCA levels were the result of their hyperinsulinemia and hyperglycemia. Our results in patients with T1DM are not incompatible with this hypothesis. Both the previously studied patients with T2DM (11) and the patients with T1DM in this study had similarly elevated A1C (9.2 ± 3.2 vs. 9.2 ± 0.1%). It is possible, for instance, that much longer periods of blood glucose elevation or normalization may be necessary to change their TF-PCA levels (12). Compatible with this notion, our patients with T1DM had higher A1Cs than the nondiabetic control subjects, reflecting chronically elevated glucose levels. Another possibility is adiponectin levels, which have been reported to be elevated in patients with T1DM (22) and which were higher in our patients compared with the nondiabetic control subjects. Inasmuch as adiponectin has been shown to decrease TF expression in human endothelial cells (23) and to inhibit adhesion of monocytic cells to endothelial cells (24), it is possible that the lack of TF-PCA stimulation by glucose and insulin in our patients with T1DM was, at least in part, related to their elevated adiponectin levels.
Moreover, insulin has been reported to inhibit TF synthesis and expression in monocytes and platelets and monocyte microparticles, and these effects were blunted in patients with T2DM because of insulin resistance (25–27). Thus, the lack of rise in TF-PCA in T1DM during combined hyperglycemia-hyperinsulinemia may be related to an inhibitory effect of insulin. The differences in TF-PCA responses to hyperglycemia-hyperinsulinemia observed between patients with T1DM and nondiabetic control subjects may also be due to the higher levels of plasma adiponectin and/or differences in sensitivity of monocytes to insulin.
Plasma FVIIa showed a small decline during the high glucose-high insulin infusions in our patients with T1DM (Fig. 2). Decreases in plasma FVIIa have been reported in states associated with monocyte activation and increased circulating microparticles (28–30). We have reported decreases in plasma FVIIa in nondiabetic patients in response to 24-h glucose and insulin infusions (13). TF is the principal ligand for FVIIa, and one potential explanation for the decline of FVIIa may be the removal from plasma of FVIIa by binding to monocytes and microparticles (which rose during the hyperglycemic-hyperinsulinemic clamps).
In summary, our studies provide evidence for increased levels of circulating TF-PCA and FVIIa in a small group of patients with T1DM, suggesting a procoagulant state that is likely to contribute to acute vascular events. They also show that the regulation of circulating TF-PCA levels in patients with T1DM and T2DM is different. The observation that TF-PCA levels are elevated in diabetes may be therapeutically important. Clearly, however, additional studies are needed to confirm our findings in a larger number of patients and to obtain insights into the reasons why the regulation of TF expression in the two forms of diabetes was different.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01-HL-073367 (to G.B. and A.K.R.) and R01-DK-066003 (to G.B.) and an American Diabetes Association grant (1-10-CT-06) (to G.B.).
No potential conflicts of interest relevant to this article were reported.
A.S. researched data and contributed to the discussions. G.B. and A.K.R. designed the study and wrote the manuscript. C.H. performed clinical studies. J.G. researched data and contributed to the discussions. A.K.R. researched data and co-wrote the manuscript. G.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Maria Mozzoli, BS, and Karen Kresge, BS, (Clinical Research Center, Temple University Hospital) for excellent technical assistance and Constance Harris Crews (Division of Endocrinology, Diabetes and Metabolism, Temple University School of Medicine) for typing the manuscript.
References
- 1.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 2.Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res 2010;7:260–273 [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Chonchol M, Zoppini G, Franchini M. Hemostatic disorders in type 1 diabetes mellitus. Semin Thromb Hemost 2011;37:58–65 [DOI] [PubMed] [Google Scholar]
- 4.Carmassi F, Morale M, Puccetti R, et al. Coagulation and fibrinolytic system impairment in insulin dependent diabetes mellitus. Thromb Res 1992;67:643–654 [DOI] [PubMed] [Google Scholar]
- 5.Rauch U, Nemerson Y. Tissue factor, the blood, and the arterial wall. Trends Cardiovasc Med 2000;10:139–143 [DOI] [PubMed] [Google Scholar]
- 6.Boganov VY, Osterund B. Cardiovascular complications of diabetes mellitus: the tissue factor perspective. Thromb Res 2010;125:112–118 [DOI] [PubMed] [Google Scholar]
- 7.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci USA 1989;86:2839–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA 1999;96:2311–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Key NS, Slungaard A, Dandelet L, et al. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood 1998;91:4216–4223 [PubMed] [Google Scholar]
- 10.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood 2004;104:3190–3197 [DOI] [PubMed] [Google Scholar]
- 11.Boden G, Vaidyula VR, Homko C, Cheung P, Rao AK. Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: effects of insulin and glucose. J Clin Endocrinol Metab 2007;92:4352–4358 [DOI] [PubMed] [Google Scholar]
- 12.Sambola A, Osende J, Hathcock J, et al. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation 2003;107:973–977 [DOI] [PubMed] [Google Scholar]
- 13.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes 2006;55:202–208 [PubMed] [Google Scholar]
- 14.Boden G, Vaidyula V, Homko C, Mozzoli M, Rao AK. Differential effects of somatostatin on circulating tissue factor procoagulant activity and protein. Am J Physiol Endocrinol Metab 2007;292:E1333–E1339 [DOI] [PubMed] [Google Scholar]
- 15.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol 1957;17:237–246 [DOI] [PubMed] [Google Scholar]
- 16.Rao AK, Vaidyula VR, Bagga S, et al. Effect aof antiplatet agents clopidogrel, spirin, and cilostazol on circulating tissue factor procoagulant activity in patients with peripheral arterial disease. Thromb Haemost 2006;96:1–6 [PubMed] [Google Scholar]
- 17.Tehrani S, Mobarrez F, Antovic A, et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res 2010;126:e225–e231 [DOI] [PubMed] [Google Scholar]
- 18.Tutar E, Ozcan M, Kilickap M, et al. Elevated whole-blood tissue factor procoagulant activity as a marker of restenosis after percutaneous transluminal coronary angioplasty and stent implantation. Circulation 2003;108:1581–1584 [DOI] [PubMed] [Google Scholar]
- 19.Amengual O, Atsumi T, Khamashta MA, Hughes GR. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemost 1998;79:276–281 [PubMed] [Google Scholar]
- 20.Asakura H, Kamikubo Y, Goto A, et al. Role of tissue factor in disseminated intravascular coagulation. Thromb Res 1995;80:217–224 [DOI] [PubMed] [Google Scholar]
- 21.Morrissey JH, Mutch NJ. Tissue factor structure and function. In Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, Eds. Philadelphia, Lippincott Williams & Wilkins, 2006 [Google Scholar]
- 22.Leth H, Andersen KK, Frystyk J, et al. Elevated levels of high-molecular-weight adiponectin in type 1 diabetes. J Clin Endocrinol Metab 2008;93:3186–3191 [DOI] [PubMed] [Google Scholar]
- 23.Chen YJ, Zhang LQ, Wang GP, et al. Adiponectin inhibits tissue factor expression and enhances tissue factor pathway inhibitor expression in human endothelial cells. Thromb Haemost 2008;100:291–300 [PubMed] [Google Scholar]
- 24.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 1999;100:2473–2476 [DOI] [PubMed] [Google Scholar]
- 25.Gerrits AJ, Koekman CA, Yildirim C, Nieuwland R, Akkerman JW. Insulin inhibits tissue factor expression in monocytes. J Thromb Haemost 2009;7:198–205 [DOI] [PubMed] [Google Scholar]
- 26.Gerrits AJ. Koekman Ca. van Haeften TW, Akkerman JW. Platelet tissue factor synthesis in type 2 diabetic patients in resistant to inhibition by insulin. Diabetes 2010;59:1487–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira IA, Mocking AI, Feijge MA, et al. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2006;26:417–422 [DOI] [PubMed] [Google Scholar]
- 28.Taylor FB, Haddad PA, Hack E, et al. Two-stage response to endotoxin infusion into normal human subjects: Correlation of blood phagocyte luminescence with clinical and laboratory markers of the inflammatory, hemostatic response. Crit Care Med 2001;29:326–334 [DOI] [PubMed] [Google Scholar]
- 29.Mesters RM, Mannucci PM, Coppola R, Keller T, Ostermann H, Kienast J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood 1996;88:881–886 [PubMed] [Google Scholar]
- 30.Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood 2004;103:4545–4553 [DOI] [PubMed] [Google Scholar]