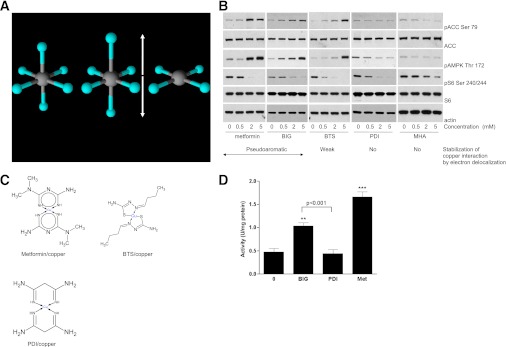
FIG. 3.
Interruption of π-electron delocalization intrinsic to biguanides results in compounds that selectively target S6 phosphorylation, lacking effects on AMPK. A: Commonly, distortion of copper d9 octahedral coordination geometry (left) results in elongated axial ligands (middle). The extreme case of axial ligand elongation is the square planar structure (right). Copper is induced to adopt this geometry when metformin binds. B and C: H4IIE cells were grown in serum-free medium for 2 h, followed by stimulation with or without the metformin analogs shown, at the concentrations shown. Lysates were prepared for immunoblotting with the antibodies indicated. The chemical structures of metformin, BTS, and PDI complexed with divalent copper are shown in C. Metformin and BIG form pseudoaromatic planar complexes with copper in a square planar geometry, characterized by fairly uniform intermediate bond lengths in the ring, stabilized by π-electron delocalization (11–14). Thiosemicarbazones such as BTS also form extensively conjugated ring structures with copper (33); however, data on bond lengths (30,31), supported by spectroscopic studies (42), indicate that the resonance form shown predominates, with reduced delocalization of electron density and significant deviation from planarity compared with biguanides (11,14,31). PDI has π-electron delocalization in the ring abolished by substitution of N3 (12,32) and is not planar. D: H4IIE cells that had been deprived of serum for 2 h were treated with 2 mM of the agents shown for 3 h (metformin [Met]), before AMPK assay as described in research design and methods. Statistical analysis was carried out as in Fig. 1D. **P < 0.01, ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)