Abstract
We have previously developed a combination therapy (CT) using anti-CD3 monoclonal antibodies together with islet-(auto)antigen immunizations that can more efficiently reverse type 1 diabetes (T1D) than either entity alone. However, clinical translation of antigen-specific therapies in general is hampered by the lack of biomarkers that could be used to optimize the modalities of antigen delivery and to predict responders from nonresponders. To support the rapid identification of candidate biomarkers, we systematically evaluated multiple variables in a mathematical disease model. The in silico predictions were validated by subsequent laboratory data in NOD mice with T1D that received anti-CD3/oral insulin CT. Our study shows that higher anti-insulin autoantibody levels at diagnosis can distinguish responders and nonresponders among recipients of CT exquisitely well. In addition, early posttreatment changes in proinflammatory cytokines were indicative of long-term remission. Coadministration of oral insulin improved and prolonged the therapeutic efficacy of anti-CD3 therapy, and long-term protection was achieved by maintaining elevated insulin-specific regulatory T cell numbers that efficiently lowered diabetogenic effector memory T cells. Our validation of preexisting autoantibodies as biomarkers to distinguish future responders from nonresponders among recipients of oral insulin provides a compelling and mechanistic rationale to more rapidly translate anti-CD3/oral insulin CT for human T1D.
Autoimmune diabetes, also known as type 1 diabetes (T1D), is the consequence of an immune-mediated loss of β-cells (1). Much effort has been devoted to understanding its etiology; nevertheless, T1D incidence has been continuously rising over the past years, especially in young children (2,3). To counter the autoimmune attack, various immune interventions are being developed (4), and many systemically acting immunotherapies are designed to reduce diabetogenic effector T cells (Teffs). Among these, non–Fc receptor (FcR) binding CD3-specific antibodies have been extensively studied and have shown clinical efficacy by preserving β-cell function in newly diagnosed patients (5,6). However, the improved β-cell function was not maintained after a single short-term treatment (7,8), indicating a recurrence of autoimmunity. In light of these clinical data, alternative strategies are needed to improve efficacy. One option is to reinject the drug when its therapeutic effect wanes, and current trials are under way to test repeated anti-CD3 treatments; however, increased doses may enhance side effects (7,9) and favor sensitization (5,6,10). As a consequence, one recent phase III clinical trial was conducted to evaluate the potency of low-dose anti-CD3 to preserve β-cell mass in new-onset T1D patients. Otelixizumab (Tolerx and GlaxoSmithKline), a humanized non–FcR binding CD3-specific antibody, unfortunately failed to preserve β-cell function when administered at a lower dose. In a similar manner, teplizumab (MacroGenics) failed to reach a more ambitious combined end point of lower HbA1c and insulin usage (11). In contrast to systemic immune modulators, antigen-specific therapies control autoimmunity locally in the islets and the pancreatic lymph nodes (PLN), thus circumventing systemic side effects (12). To improve efficacy and avoid sensitization, we combined short-course anti-CD3 treatment with β-cell autoantigen administration in newly diabetic NOD mice (13). While this strategy successfully induced remission, a critical gap to efficiently translate this combination therapy (CT) to the clinic is to develop suitable biomarkers that would aid in optimizing immunization (dose, route, and frequency) and defining individuals most likely to benefit from the CT. We used a validated mathematical model of murine T1D pathophysiology (T1D PhysioLab platform) (14,15) for defining suitable biomarkers that can differentiate responders from nonresponders predictively and that are practical for use in human trials. We show that animals, who best responded to this CT, had elevated anti-insulin autoantibodies (IAAs) before therapy and lower circulating proinflammatory cytokines after treatment initiation. Moreover, we demonstrate that repeated insulin feedings ameliorate and sustain the efficacy of low-dose anti-CD3 therapy when started after new onset. The combined approach possesses the unique ability to dampen long-term reactivation of memory Teffs through sustained action of insulin-specific regulatory T cells (Tregs). Both treatments have a long history in clinical trials, and their safety profiles are well established (5,6,16), which should hopefully facilitate future clinical translation of this CT.
RESEARCH DESIGN AND METHODS
Biosimulations.
The Entelos T1D PhysioLab platform, a validated mathematical model of T1D pathophysiology in NOD mice (14,15), was used to identify candidate biomarkers for anti-CD3/oral insulin CT. Technically, a customized cohort of 93 virtual NOD mice was generated. Each virtual mouse is an alternate parameterization of the mechanistic representation and therefore represents alternate hypotheses on the relative roles of different pathways in the underlying pathophysiology. Each virtual mouse simulates disease progression to hyperglycemia and was tested against 10 immunointerventions to confirm consistency with published behaviors of NOD mice (15). The cohort was calibrated to match the mean onset time of T1D and remission rate (± SD) in response to CT or monotherapies for NOD mice housed at the La Jolla Institute for Allergy and Immunology (LIAI).
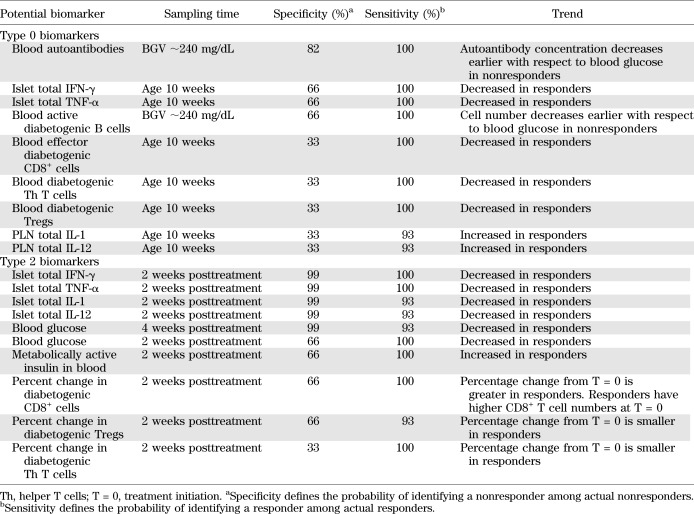
More than 20 potential biomarkers represented in the platform were identified and evaluated based on publicly available literature for potential translation to serum biomarkers.
To identify pretreatment (type 0) biomarkers, we evaluated potential biomarker levels at various pretreatment time points and then applied discriminant function analysis to identify the biomarker(s) with the best specificity (probability of predicting a nonresponder among actual nonresponders) and sensitivity (probability of predicting a responder among actual responders) for remission. To identify early posttreatment (type 2) biomarkers, we evaluated potential biomarker levels at various early posttreatment time points and then applied discriminant function analysis to identify biomarker(s) with the best specificity and sensitivity.
Mice.
NOD/LtJ female mice were purchased from The Jackson Laboratory (Bar Harbor, ME), maintained at the LIAI under pathogen-free conditions, and handled in accordance with protocols approved by the organization’s animal care committee.
Treatments.
After new-onset diabetes (blood glucose values [BGV] >250 mg/dL), NOD mice were treated 3 consecutive days intravenously at 1, 2.5, 5, 10, 15, or 25 μg/day with the non–FcR binding anti–CD3-ε F(ab’)2 (clone 145–2C11; Bio X Cell). This treatment was given alone or in combination with porcine insulin (I5523–50MG; Sigma-Aldrich). Insulin was diluted in acidified PBS1× (pH = 2.5) at 2.5 mg/mL and administered orally (starting day 0 twice a week for 5 consecutive weeks) at 1 mg/day by gastric intubation.
Isolation of intrapancreatic lymphocytes.
Isolation of intrapancreatic lymphocytes was performed as previously described (17).
Enzyme-linked immunosorbent spot assay and enzyme-linked immunosorbent assay.
Interleukin (IL)-10, IL-4, and interferon (IFN)-γ produced by isolated lymphocytes after stimulation with insulin B (insB)9–23 peptide (10 μg/mL) or porcine insulin (20 μg/mL) were detected by enzyme-linked immunosorbent spot assay as described (18). For enzyme-linked immunosorbent assay, lymphocytes were extracted from various organs of NOD mice. CD8-depleted lymphocytes (5 × 105 cells/well) were cultured in 96-well microtiter plates and stimulated with anti-CD3 antibody clone 145–2C11 (0.5 μg/mL). Culture supernatants were harvested after 72 h, and mouse transforming growth factor (TGF)-β1 concentrations measured by ELISA (660.050.096; Cell Sciences).
Adoptive cell transfers.
To assess the diabetogenic potential of effector memory T cells (Tems), total T cells from spleen or PLN/pancreas of protected NOD mice were transferred into female NOD-SCID (severe combined immunodeficient) recipient mice (106 cells/recipient). In other experiments, total CD4+ or CD4+CD25+ cells were purified by negative selection from splenocytes and PLNs of protected animals (114–16D from Invitrogen/Life Technologies; and CD4+CD25+ Treg isolation kit from Miltenyi Biotec). Eight-week-old female NOD-SCID recipient mice received intravenously 5 × 105 diabetogenic cells alone or together with purified CD4+ (3 × 106 cells/mouse) or CD4+CD25+ (1 × 106 cells/mouse) T cells.
Mouse IAA assay.
Serum was collected from newly diabetic female NOD mice before treatment randomization, and IAAs were analyzed at the Barbara Davis Center, University of Colorado Denver, as described previously (19).
Serum cytokine analyses.
Serum was collected 2 weeks after treatments started and kept at −40°C until further analysis. Concentrations of cytokines were measured by using Bio-Plex luminex assay (12-Plex Pro Mouse cytokine group 1; Bio-Rad).
Statistical analysis.
Data analysis was performed using GraphPad Prism 4.00. Survival curves were computed using the Kaplan-Meier method. For other in vivo data, the significance was evaluated using a two-way ANOVA test. Statistical significance for other data was measured using an unpaired two-tailed Mann-Whitney U test.
RESULTS
Combination of in silico biosimulations and wet-laboratory studies identifies surrogate biomarkers.
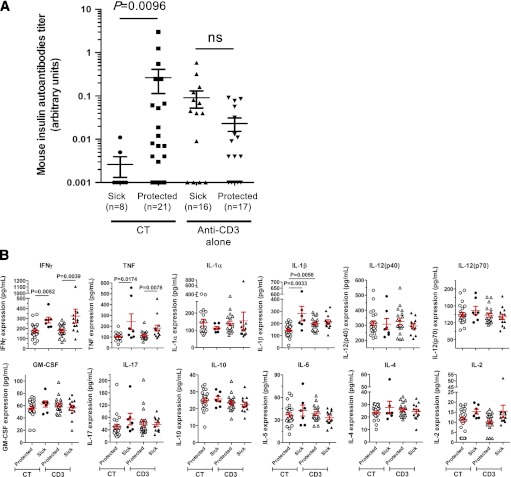
Clinical translation of antigen-specific therapies in general is hampered by the lack of biomarkers. We decided to build on previous work (13) and determine whether therapeutic efficacy of oral insulin combined with a short-term low-dose anti-CD3 antibody therapy can be predicted in NOD mice. A suitable biomarker should be measurable in peripheral blood to be applicable for clinical trials. However, the combination of small sample volumes, numerous potential candidates, and sampling times makes their preclinical selection challenging. To circumvent these issues, we questioned a validated mathematical model of T1D pathophysiology, the T1D PhysioLab platform (14,15). We simulated experimental therapeutic protocols in a cohort of virtual NOD mice that recapitulate the timing of diabetes onset observed in our experimental colony. Simulation results for >20 potential biomarkers (Supplementary Fig. 1) were analyzed to determine their ability to distinguish responders from nonresponders prior to treatment (type 0 biomarkers) or to provide an early posttreatment indicator for long-term therapeutic outcome (type 2 biomarkers). Our biosimulations predicted that elevated autoantibody levels at time of diagnosis would best differentiate responders from nonresponders to CT (responders being identified as NOD mice with BGV <250 mg/dL at 15 weeks after treatment initiation) (Table 1). To test this prediction in the wet laboratory, IAA levels were assessed in serum samples collected from newly diabetic NOD mice before treatment randomization. Data revealed that mice with higher pretreatment levels of serum IAAs responded with a much higher likelihood to CT but not anti-CD3 monotherapy (Fig. 1A). Wet-laboratory data are fully consistent with the in silico predictions and the hypothesis that IAAs may indicate a better capability to induce insulin-specific Tregs after oral insulin immunization.
TABLE 1.
Type 0 and type 2 biomarkers identified by in silico biosimulations
FIG. 1.
Circulating biomarkers predict long-term efficacy of anti-CD3 antibody/oral insulin CT. A: Mouse IAA levels are efficient type 0 biomarkers. After new-onset of T1D, NOD females were randomized to receive anti-CD3 treatment with or without oral insulin. Before treatment randomization, the serum of each individual NOD mouse was collected to measure IAA levels in a blinded fashion. At 15 weeks posttreatment, NOD mice with a BGV <250 mg/dL were considered protected. Data show the results obtained in individual mice (n = 8–21 per group). ns, P > 0.05. B: Serum levels of pro- and anti-inflammatory cytokines in treated NOD mice. Sera from NOD mice treated with anti-CD3 alone or anti-CD3/oral insulin (CT) were collected 2 weeks after treatment started. The concentration of each cytokine was measured by Bioplex assay. At 15 weeks posttreatment, NOD mice with a BGV <250 mg/dL were considered protected. Data show the results obtained in individual mice (n = 7–23 per group). GM-CSF, granulocyte-macrophage colony-stimulating factor.
Biosimulations also predicted that decreased proinflammatory cytokine levels 2 weeks after CT initiation were indicative of long-term remission 15 weeks after treatment initiation (Table 1). Laboratory experiments confirmed the in silico predictions for circulating IFN-γ, tumor necrosis factor (TNF)-α, and IL-1β but not IL-12 (Fig. 1B). However, the overlap between the cytokine concentrations from responders and nonresponders of CT would challenge the accuracy of these values in predicting efficacy in individual mice. Therefore, we evaluated the ability of a combination of circulating IFN-γ, TNF-α, and IL-1β cytokines to predict responders from nonresponders (Supplementary Table 1). The average values for each cytokine were calculated with all responder and nonresponder mice (IFN-γ = 203 pg/mL; TNF-α = 137 pg/mL; IL-1β = 177 pg/mL). In total, 78.3% of responders showed two or three cytokines with an individual value below the average value as compared with only 14.3% of nonresponder mice. Therefore, a combination of serum cytokines might be needed to maximize their predictive value.
In a similar manner, the average serum levels of IFN-γ and TNF-α were found diminished in anti-CD3–treated NOD mice showing long-standing disease remission. Together, these results provide crucial candidate biomarkers to move this CT to the clinic.
Oral insulin potentiates the anti-CD3–mediated reversion of new-onset T1D in NOD mice.
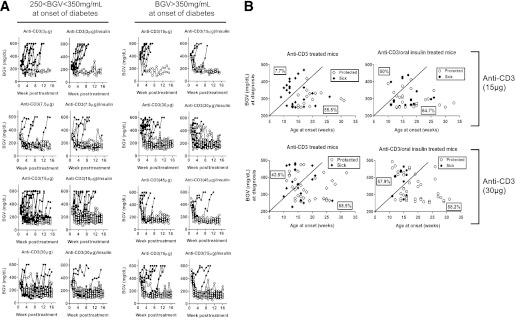
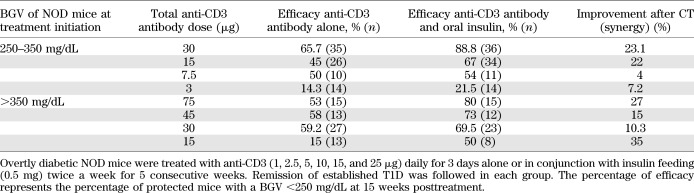
A detailed dose-response study determined the minimum effective dose to obtain synergy between anti-CD3 and oral insulin. We show that synergy is highly dependent on the loss of insulin production capacity at treatment initiation coupled with the precise anti-CD3 dose (Fig. 2A and Table 2). Animals with the lowest β-cell function at diagnosis, characterized by high BGV, profited most from a higher dose of anti-CD3 given as monotherapy (Fig. 2B). Within this group (BGV >350 mg/dL), the lowest dose of antibody (15 μg) was unable to reverse T1D. In contrast, when BGVs were between 250 and 350 mg/dL at onset, reflective of comparatively higher remaining β-cell function, the 15-μg dose reversed T1D in 45% of the mice (Table 2). However, lowering the total dose to 3 μg resulted in a significant loss of protection. These data are consistent with a previous study (20) and define a minimum-dose threshold below which anti-CD3 antibody treatment is inefficient at reversing overt T1D. This threshold varies depending on the disease status (identified by BGV) at clinical onset.
FIG. 2.
Oral insulin immunization is required to reverse severe T1D with low-dose anti-CD3. A: BGVs of individual NOD mice treated after new-onset T1D with CD3-specific antibody alone or in combination with oral insulin. Newly diagnosed diabetic female NOD mice were randomized into 12 groups to receive 3, 7.5, 15, 30, 45, or 75 μg anti-CD3 antibody alone or in combination with oral insulin. The symbols represent the BGVs of each individual mouse for up to 16 weeks after treatment started (n = 8–36 per group). Open and closed circles discriminate protected and non-protected animals at 16 weeks posttreatment initiation. B: Overtly diabetic NOD mice were treated with anti-CD3 (5 or 10 μg) daily for 3 days alone or in conjunction with insulin feedings (0.5 mg) twice a week for 5 consecutive weeks. Remission of established T1D was followed in each group. Mice were considered protected if their BGV was <250 mg/dL at 15 weeks posttreatment. The BGV and age at onset of each individual NOD mouse treated with either 30 or 15 μg anti-CD3 antibody in combination or not with oral insulin are plotted on the graphs (n = 35–62 mice per group). Mice with the most severe T1D (characterized by an early onset and higher BGVs at diagnosis) are distinguished from mice with less severe T1D by an arbitrary dark line. The percentage of responders within the most severe or less severe T1D groups at diagnosis are calculated and displayed on each side of the arbitrary dark line.
TABLE 2.
Remission rates of newly diabetic NOD mice after anti-CD3 antibody or anti-CD3/oral CTs
Addition of oral insulin significantly improved the clinical outcome of anti-CD3 treatment in most groups, and a 10–35% increased remission rate was observed after CT (Fig. 2A and Table 2). When BGVs were between 250 and 350 mg/dL, a long-term remission rate of up to 88.8% was obtained after CT as compared with 65.7% with anti-CD3 alone. A high remission rate (∼80%) was also seen in the group receiving the CT when BGV was >350 mg/dL at onset, but the total anti-CD3 dose needed to be increased to 75 μg (13,21,22). Synergy was particularly striking in the most severe diabetes cases characterized by an early onset and greater loss of β-cell function at diagnosis (Fig. 2B). Although synergy was observed over a wide range of CD3-specific antibody doses, our data indicate that there might be a critical threshold below which anti-CD3 is unable to synergize with oral insulin immunization.
Modulation of the CD3/T-cell receptor complex and lymphocyte cell counts.
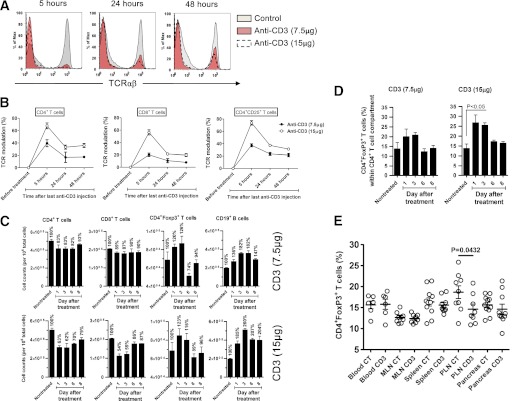
To better understand the loss of synergy at lower anti-CD3 doses, we compared the antigenic modulation of the CD3/T-cell receptor (TCR) complex in NOD mice treated with 7.5 μg (no synergy) or 15 μg (synergy) anti-CD3. Both doses rapidly induced a transient reduction of TCR-αβ+ T cells in peripheral blood (Fig. 3A), possibly as a result of sequestration rather than depletion of T cells as suggested before (23–25). The synergy obtained after a 15-μg anti-CD3 dose was associated with strong TCR modulation of CD4+, CD8+, and CD4+CD25+ T-cell populations ranging from ∼60 to 80% (Fig. 3B). In contrast, the nonsynergistic dose of 7.5 μg led to a modest TCR modulation of ∼40% on CD4+ cells and only ∼20% for CD8+ cells (Fig. 3B), arguing that CD8+ T cells are more resistant than CD4+ T cells to TCR modulation after anti-CD3 treatment. Our observations suggest that an anti-CD3 antibody dose leading to strong TCR modulation (>50%) is required to allow for synergy with oral insulin to occur.
FIG. 3.
CD3 modulation is required for synergy between anti-CD3 and oral insulin treatments. A and B: In vivo TCR-αβ modulation 5, 24, and 48 h after injection with non–FcR binding CD3-specific antibody, 145–2C11 F(ab’)2, at 2.5 or 5 μg/day for 3 consecutive days. Data are mean ± SEM of n = 6 individual mice per group. C: The absolute number (± SEM) of CD4+, CD8+, CD4+Foxp3+, and CD19+ cells was measured in the PLN of NOD mice treated with non–FcR binding CD3-specific antibody, 145–2C11 F(ab’)2, at 2.5 or 5 μg/day for 3 consecutive days. D: Mean proportion (± SEM) of CD4+Foxp3+ T cells in the PLN was analyzed by fluorescence-activated cell sorter on days 0 (nontreated), 1, 3, 6, and 8 after non–FcR binding anti-CD3 treatment (2.5 or 5 μg/day for 3 consecutive days). Data are means ± SEM of two independent experiments with at least three mice per time point. E: Mean proportion (± SEM) of CD4+Foxp3+ T cells in various organs at 2 weeks posttreatment with 30 μg anti-CD3 antibody alone (CD3) or in combination with oral insulin (CT). Data show the results obtained in individual mice. n = 6–12 in one representative experiment of three. MLN, mesenteric lymph node.
We next measured the absolute number of lymphocytes in the PLN of NOD mice treated with anti-CD3 antibody. The higher dose allowing for synergy with oral insulin to occur (15 μg) was associated with a greater reduction in CD4+ and CD8+ cell numbers but, as described recently (26), no difference in CD4+Foxp3+ Tregs (Fig. 3C). Our findings suggest that functional TCR modulation and physical removal or redirection (debulking) (10) of CD4+ and CD8+ cells, leading to a numeric reduction of pathogenic T cells at the site of inflammation, is necessary for optimal synergy between anti-CD3 and oral insulin to develop. The net result also is that Tregs comprise a greater proportion of the CD4+ population after synergistic treatment (Fig. 3D). Finally, we quantified the proportion of Tregs in various organs after both treatments (Fig. 3E). The proportion was increased only in the PLNs of mice receiving CT.
Tem and Treg numbers are regulated by anti-CD3/oral insulin CT.
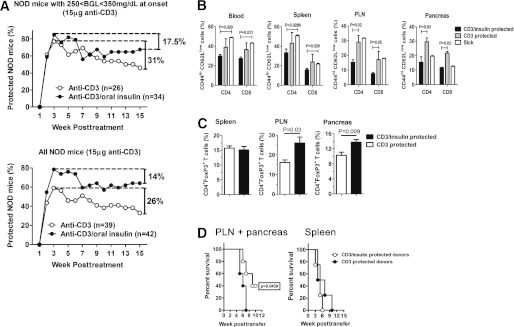
We next determined whether efficacy of low-dose anti-CD3 alone or in combination with oral insulin was long lasting. A large proportion of NOD mice treated with low-dose anti-CD3 monotherapy (15 μg total) rapidly reverted to normoglycemia but relapsed over the course of the study to reach 33–46% protection (depending on the BGV at onset) at 15 weeks posttreatment (Fig. 4A). In contrast, protection afforded by CT was higher than anti-CD3 alone and more sustained with ∼65% protection at the end of the study. Thus, oral insulin treatment was able to reduce T1D recurrence rate after low-dose anti-CD3 antibody treatment (26–31 vs. 14–17.5% recurrence after anti-CD3 or CT, respectively) (Fig. 4A).
FIG. 4.
Oral insulin synergizes with anti-CD3 therapy to prolong long-term remission from T1D by sustaining intrapancreatic Tregs and controlling Tems. A: Overtly diabetic NOD mice were randomized to receive 15 μg CD3-specific F(ab’)2 fragment alone or in combination with oral insulin (0.5 mg twice a week for 5 consecutive weeks). The percentage of protected mice was followed in each group, and the data are presented in two separate graphs for 1) mice with a BGV between 250 and 350 mg/dL at onset or 2) the entire mouse cohort independently of BGV at onset (n = 26–42 mice per group). B: The percentage of CD44highCD62Llow Tems within the CD4 or CD8 compartments was calculated in various organs of NOD mice either diabetic (sick) or protected for at least 15 weeks by treatment with 15 μg anti-CD3 alone (CD3) or in combination with oral insulin (CD3/insulin). Data are expressed as means ± SEM and are representative of three independent experiments with n = 4–8 mice per group. C: The percentage of CD4+Foxp3+ cells within the CD4 population was measured in various organs of protected NOD mice 15 weeks posttreatment with 15 μg anti-CD3 alone or in combination with oral insulin. Data are expressed as means ± SEM and are representative of three independent experiments with n = 5–9 mice per group. D: Total T cells were purified from the spleens or pooled PLNs and pancreata of donor NOD mice protected for at least 15 weeks by a treatment with 15 μg anti-CD3 alone or in combination with oral insulin. Equal numbers of total T cells (106 cells) were transferred into NOD-SCID recipient mice, and diabetes development (BGVs >250 mg/dL) was followed. Data are representative of two independent experiments with n = 6–8 mice per group.
The recurrence of T1D in mice treated with anti-CD3 alone could have been mediated by Tems, which are involved in maintaining autoimmunity and are also responsible for the recurrence of T1D after pancreas or islet transplantation (27,28). Therefore, we assessed the effect of CT on pathogenic Tems by evaluating the proportion of CD44highCD62Llow Tems within different organs of NOD mice undergoing long-term remission. The proportions of both CD4+ and CD8+ Tems were more reduced in the PLN and pancreas of CT-treated NOD mice compared with those receiving only anti-CD3 monotherapy (Fig. 4B).
In addition, long-term protection after CT also can be explained by a higher proportion of CD4+Foxp3+ Tregs in the PLN and pancreas, but not spleen, of CT-treated NOD mice as compared with anti-CD3 monotherapy (Fig. 4C). These results suggest that CT is superior compared with low-dose anti-CD3 treatment by maintaining a higher Treg-to-Tem ratio within the pancreas and PLN. Consistent with these findings, T cells purified from PLN and pancreas of CT-treated mice were less diabetogenic than those recovered from NOD mice protected with anti-CD3 alone as shown by adoptive transfers into NOD-SCID recipients (Fig. 4D).
Suppressive capacity of islet-specific Tregs after anti-CD3 or anti-CD3/oral insulin treatments.
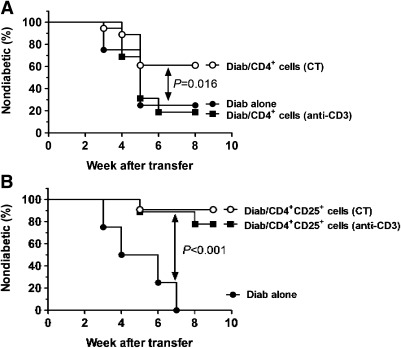
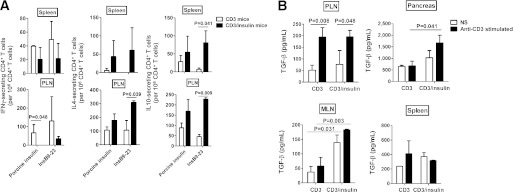
To examine whether functional insulin-specific Tregs were induced by the anti-CD3/oral insulin CT, we first compared the suppressive capacity of Tregs isolated from NOD mice protected after anti-CD3 monotherapy or CT. Splenocytes from diabetic NOD mice were adoptively transferred alone, or in combination with CD4+ or CD4+CD25+ T cells purified from treated NOD mice, into NOD-SCID recipient mice. CD4+ T cells purified from CT but not anti-CD3 monotherapy–treated NOD mice induced significant protection from diabetes (60 vs. 22% protection, P = 0.016) (Fig. 5A). Adoptive transfer of CD4+CD25+ cells derived from NOD mice treated with either anti-CD3 alone or CT afforded a similar 80–90% protection level that was higher than protection achieved with total CD4+ cells (Fig. 5B), presumably because most CD4+CD25+ cells are Foxp3+ Tregs. These results support the hypothesis that CT augments the number of insulin-specific T cells with regulatory properties within the CD4 population. Consistent with this, in vitro stimulation of PLN CD4+ T cells from CT-treated mice with insB9–23 peptide demonstrated increased frequency of cells secreting regulatory IL-4 and IL-10 cytokines (Fig. 6A). Because tolerance induction by anti-CD3 monotherapy is TGF-β–mediated (21), we also assessed TGF-β production by purified T cells after either anti-CD3 alone or CT. As previously observed (21), TGF-β expression by T cells was significantly increased after anti-CD3 therapy (Fig. 6B). CT increased the production of soluble TGF-β by CD4+ cells in the mesenteric lymph nodes and pancreas. Previous work from Tonkin and Haskins’ (29) laboratory had shown that intrapancreatic Tregs suppress T1D by inhibiting infiltrating Teffs and macrophages through a TGF-β–dependent mechanism (29). Therefore, the significant augmentation of TGF-β after CT could represent one mechanism by which CT-induced Tregs better control T1D.
FIG. 5.
Synergy of anti-CD3 and oral insulin therapies augments the number of insulin-specific CD4+ T cells with in vivo suppressive capacities. Purified CD4+ (A) or CD4+CD25+ cells (106 cells) (B) from NOD mice treated with anti-CD3 alone or anti-CD3/oral insulin (CT) and diabetogenic cells (Diab; 3 × 106 cells) from fully diabetic NOD mice were cotransferred into NOD-SCID recipient mice. Autoimmune diabetes development and kinetics were followed in each group. Data are representative of two independent experiments with n = 7–8 mice per group.
FIG. 6.
Oral insulin in combination with anti-CD3 therapy increases anti-inflammatory cytokine levels expressed by insulin-specific T cells. A: Cytokine secretion by insulin-specific CD4+ T cells. Spleens and PLNs of protected NOD mice were collected at 4 weeks posttreatment with anti-CD3 antibody alone (CD3) or in combination with oral insulin (CD3/insulin). The number of IL-4, IL-10, and IFN-γ–expressing CD4+ T cells was assessed by enzyme-linked immunosorbent spot assay after in vitro stimulation with or without porcine insulin (20 μg/mL) or insB9–23 peptide (10 μg/mL). Data are expressed as means ± SEM of three independent experiments. B: CD3/oral insulin CT augments TGF-β expression by CD4+ T cells. Lymphocytes were recovered from various organs of NOD mice at 4 weeks posttreatment with anti-CD3 alone or in combination with oral insulin. The amount of TGF-β was measured by enzyme-linked immunosorbent assay in the supernatant of CD8-depleted cells with or without anti-CD3 stimulation. Histograms show the means ± SEM of two independent experiments. NS, nonstimulated.
DISCUSSION
Efficacy of CD3-specific antibodies has been suboptimal in T1D clinical trials (5,6,8,11,30). Here, we performed a comprehensive preclinical study to predict, enhance, and prolong the efficacy of anti-CD3–mediated remission of overt T1D by combining it with oral insulin treatment. Our results identified circulating IAA levels as a potent surrogate biomarker for predicting responders to CT. Our data in NOD mice are consistent with human data obtained in a post hoc analysis of the Diabetes Prevention Trial of T1D (DPT-1) showing that patients with the highest IAA levels profited most from oral insulin treatment (31). Whether these IAAs are only a marker of preexisting insulin-reactive T cells quickly responding to the CT or participate in the presentation of their cognate antigen to the immune system as observed with other islet autoantigens (32) remains to be determined. Since exogenous insulin therapy in T1D patients will produce IAAs that are indistinguishable from preexisting natural IAAs, it will be required to perform a blood draw quickly after diagnosis (before new IAAs develop) to determine preexisting circulating IAA levels. A recent study (33) confirms previous observations that higher levels of preexisting IAAs correlate with a more aggressive autoimmune destruction of β-cells. Therefore, patients with more aggressive T1D might profit better from our CT. We also show that animals who best responded to CT had lower circulating proinflammatory cytokines after treatment initiation and, thus, a combination of serum cytokines might be needed to maximize their predictive value.
Since T1D mainly affects young individuals, clinicians are hesitant to augment the anti-CD3 dose and increase potential adverse reactions. Therefore, much effort should be placed on improving CT using a lower anti-CD3 dose. Our data clearly show that feeding insulin to newly diabetic NOD mice is a safe and efficient strategy to strengthen clinical efficacy of low-dose anti-CD3 antibody treatment and sustain long-term remission.
In humans, after an anti-CD3–mediated extension of the honeymoon phase in newly diabetic patients, autoimmune-driven recurrence of β-cell destruction is observed in most treated patients (5,6,10,30), especially when a lower dose of the drug is used (11). This phenomenon is well known in the transplantation field when immunosuppressants are withdrawn or reduced and results from the endogenous reactivation of autoreactive Tems, which will then progressively destroy the graft (28,34). The long-term effect of a short-course anti-CD3 antibody treatment on the recurrence of Tems had not been studied until now. The main reason is that previously defined optimal doses of anti-CD3 F(ab’)2 antibody (∼40–50 μg/day; 5 consecutive days) induced permanent remission without recurrence of autoimmunity in NOD mice (13,21,22). However, this optimal dosage for NOD mice was not mimicking the clinical outcome observed in anti-CD3–treated patients. Therefore, we performed a dose de-escalation study to determine a minimal effective dose of anti-CD3 F(ab’)2 antibody (5 μg/day; 3 consecutive days) inducing only transient preservation of β-cells in ∼60–80% of the mice followed by a slow recurrence of autoimmunity in ∼26–31% of such transiently protected animals (Fig. 4A). This low-dose anti-CD3 leads to fluctuations in β-cell function (measured by BGV) more closely resembling those observed after anti-CD3 treatment in patients (measured by circulating C-peptide levels). We used this preclinical model of T1D recurrence in NOD mice (T1Drec/NOD model) to address whether addition of oral insulin to anti-CD3 treatment could 1) augment the efficacy and 2) reduce the T1D recurrence rate in protected animals. We show that in our T1Drec/NOD model, the percentage of Tems infiltrated in the pancreas and PLN at 15 weeks after treatment was comparable to that found in newly diabetic NOD mice (Fig. 4B). Previous studies in T1D patients show an increase in the GAD65- and insB chain–specific Tem frequency after a short-course therapy with a humanized anti-CD3 antibody (35). Therefore, T1D recurrence after short-course anti-CD3 treatments in the T1Drec/NOD model or in humans seems to be the consequence of an expansion of intrapancreatic islet–specific Tems. Their proportion significantly dropped when oral insulin was coadministered with anti-CD3 in our T1Drec/NOD model, which consequently augmented the efficacy and prolonged the clinical benefits of the CT. Our data show that Tems are better controlled after CT as a result of an expansion of insulin-specific Tregs. Although short-course treatments with anti-CD3 antibodies have been described to augment the proportion of CD4+CD25+Foxp3+ Tregs (36), we demonstrated here that an increased proportion of total Tregs after anti-CD3 antibody treatment does not solely explain the synergy with oral insulin (Fig. 3C). Our dose de-escalation study revealed that synergy is lost only when anti-CD3 therapy does not transiently reduce the proportion of Teffs in the PLN.
The efficacy with oral insulin (∼60–80% protection) is far greater than any other islet-specific antigen tested to date in combination with anti-CD3 antibody (∼40–50% protection) (13,18), even at a 6- to 13-fold lower anti-CD3 dose. Two nonmutually exclusive hypotheses could explain this. First, an optimal oral insulin dose/treatment schedule has been identified with the T1D PhysioLab platform (data not shown). This in silico approach might be crucial for future clinical trials to determine under which conditions (dose and treatment schedule) an autoantigen-specific vaccine needs to be administered to mount a tolerogenic rather than immunogenic response (37). Second, in addition to the dose of antigen, the route of administration is an essential parameter to be considered for optimizing immune responses leading to tolerance induction (38). When compared with our previous CT using intramuscular GAD65 vaccine or nasal delivery of proinsulin peptide (13,18), the present work used oral delivery. Of interest, recent data from Waldron-Lynch et al. (39) show that anti-CD3 treatment induces the migration of Tregs to the small intestine in the gut through the induction of CCR6 on CD4+ T cells. If we extrapolate this scenario to our CT, anti-CD3 treatment would transiently increase the pool of Tregs localized in the gut where insulin is presented to the immune system after oral administration. This might explain the increased synergy observed with the present CT.
Thus far, preclinical studies using monoclonal anti-CD3 antibodies have been performed with a hamster anti-mouse CD3-ε–specific antibody (145–2C11). Indeed, humanized anti-CD3-ε–specific antibodies used to treat T1D in patients are not cross-reacting with the mouse CD3-ε molecule. As a consequence, these humanized antibodies were never tested preclinically in mouse models to screen for safety, clinical efficacy, and optimization of therapeutic usage. This has been a major hurdle to optimize the clinical translation of humanized anti-CD3-ε–specific antibodies from bench to bedside. A recent study describes the generation of a NOD mouse model (the NODhuCD3-ε) expressing both the human and mouse CD3-ε molecules on T cells (40). Such a humanized NOD mouse model provides the unique opportunity to test whether synergy between humanized anti-CD3 antibodies and oral insulin treatments can be obtained and to determine the optimal doses achieving maximum efficacy (41).
To conclude, we demonstrate that combining repeated insulin feedings with short-term low-dose anti-CD3 treatment safely augments and prolongs the treatment efficacy of new-onset T1D. In silico modeling is a potent approach to identify surrogate biomarkers and predict efficacy after anti-CD3/oral insulin CT. Our data show that IAA levels at diagnosis can distinguish responders from nonresponders among recipients of oral insulin therapy. Synergy requires a minimal anti-CD3 dose to rapidly and selectively delete activated pathogenic Teffs and open a temporal window to boost expansion of insulin-specific Tregs mediated by oral insulin therapy, which in return control Tems for the long term. This report provides crucial preclinical data that strongly support and facilitate moving anti-CD3/oral insulin CT from bench to bedside.
ACKNOWLEDGMENTS
This work was supported by the Juvenile Diabetes Research Foundation (Grant 36-2008-921 to D.B.) and the Brehm Coalition. Entelos Inc. received an American Diabetes Association development grant for developing the T1D PhysioLab platform. No other potential conflicts of interest relevant to this article were reported.
A.A.M., Y.Z., J.R.C., C.L.S., and L.K.M.S. performed the in silico biosimulations using the PhysioLab platform and wrote the manuscript. Y.M. and W.L. performed the experiments. M.v.H. provided important advice for the completion of the study and edited the manuscript. D.B. conceived and performed the experiments, supervised and obtained funding for the project, analyzed data, and wrote and edited the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages. M.v.H. and D.B. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to thank Gene Napolitano and Richard Ho from Entelos Inc. for their input on the project. The authors also thank Drs. Richard Flavell from the Yale School of Medicine and Cristina Penaranda and Jeffrey A. Bluestone from the University of California San Francisco for providing them with IL-10-GFP reporter NOD mice. The authors thank Liping Yu from the University of Colorado Denver for IAA measurements and are indebted to Malina McClure from LIAI for expert technical support with mouse breeding and screening and Priscilla Colby from the LIAI for excellent administrative assistance. This is manuscript number 1416 of the LIAI.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1304/-/DC1.
REFERENCES
- 1.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 3.Dahlquist GG, Nystrom L, Patterson CC, Swedish Childhood Diabetes Study Group; Diabetes Incidence in Sweden Study Group Incidence of type 1 diabetes in Sweden among individuals aged 0–34 years, 1983–2007: An analysis of time trends. Diabetes Care 2011;34:1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity 2010;32:488–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 6.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 7.Herold KC, Gitelman S, Greenbaum C, et al. Immune Tolerance Network ITN007AI Study Group Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009;132:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 2010;53:614–623 [DOI] [PubMed] [Google Scholar]
- 9.Keymeulen B, Candon S, Fafi-Kremer S, et al. Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood 2010;115:1145–1155 [DOI] [PubMed] [Google Scholar]
- 10.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 2007;7:622–632 [DOI] [PubMed] [Google Scholar]
- 11.Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peakman M, von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes 2010;59:2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresson D, Togher L, Rodrigo E, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 2006;116:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fousteri G, Chan JR, Zheng Y, et al. Virtual optimization of nasal insulin therapy predicts immunization frequency to be crucial for diabetes protection. Diabetes 2010;59:3148–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoda L, Kreuwel H, Gadkar K, et al. The Type 1 Diabetes PhysioLab platform: a validated physiologically based mathematical model of pathogenesis in the non-obese diabetic mouse. Clin Exp Immunol 2010;161:250–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pozzilli P, Pitocco D, Visalli N, et al. IMDIAB Group No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). Diabetologia 2000;43:1000–1004 [DOI] [PubMed] [Google Scholar]
- 17.Bresson D, Fousteri G, Manenkova Y, Croft M, von Herrath M. Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. J Autoimmun 2011;37:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bresson D, Fradkin M, Manenkova Y, Rottembourg D, von Herrath M. Genetic-induced variations in the GAD65 T-cell repertoire governs efficacy of anti-CD3/GAD65 combination therapy in new-onset type 1 diabetes. Mol Ther 2010;18:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta DS, Christmas RA, Waldmann H, Rosenzweig M. Partial and transient modulation of the CD3-T-cell receptor complex, elicited by low-dose regimens of monoclonal anti-CD3, is sufficient to induce disease remission in non-obese diabetic mice. Immunology 2010;130:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003;9:1202–1208 [DOI] [PubMed] [Google Scholar]
- 22.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 1994;91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch R, Eckhaus M, Auchincloss H, Jr, Sachs DH, Bluestone JA. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol 1988;140:3766–3772 [PubMed] [Google Scholar]
- 24.Hirsch R, Gress RE, Pluznik DH, Eckhaus M, Bluestone JA. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol 1989;142:737–743 [PubMed] [Google Scholar]
- 25.Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 2003;3:123–132 [DOI] [PubMed] [Google Scholar]
- 26.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol 2011;187:2015–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velthuis JH, Unger WW, van der Slik AR, et al. Accumulation of autoreactive effector T cells and allo-specific regulatory T cells in the pancreas allograft of a type 1 diabetic recipient. Diabetologia 2009;52:494–503 [DOI] [PubMed] [Google Scholar]
- 28.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE 2008;3:e2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonkin DR, Haskins K. Regulatory T cells enter the pancreas during suppression of type 1 diabetes and inhibit effector T cells and macrophages in a TGF-beta-dependent manner. Eur J Immunol 2009;39:1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skyler JS, Type 1 Diabetes TrialNet Study Group Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci 2008;1150:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes 2000;49:1621–1626 [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Herold K, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab selectively suppresses specific islet antibodies. Diabetes 2011;60:2560–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilbrands R, Huurman VA, Gillard P, et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes 2009;58:2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cernea S, Herold KC. Monitoring of antigen-specific CD8 T cells in patients with type 1 diabetes treated with antiCD3 monoclonal antibodies. Clin Immunol 2010;134:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatenoud L. Immune therapy for type 1 diabetes mellitus—what is unique about anti-CD3 antibodies? Nat Rev Endocrinol 2010;6:149–157 [DOI] [PubMed] [Google Scholar]
- 37.Fousteri G, von Herrath M, Bresson D. Mucosal exposure to antigen: cause or cure of type 1 diabetes? Curr Diab Rep 2007;7:91–98 [DOI] [PubMed] [Google Scholar]
- 38.Larché M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med 2005;11(Suppl.):S69–S76 [DOI] [PubMed] [Google Scholar]
- 39.Waldron-Lynch F, Henegariu O, Espluggues E, et al. Tepluzimab induces human gut-tropic regulatory cells in humanized mice and patients. Sci Transl Med 2012;4:118ra12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn C, You S, Valette F, et al. Human CD3 transgenic mice: preclinical testing of antibodies promoting immune tolerance. Sci Transl Med 2011;3:68ra10. [DOI] [PubMed] [Google Scholar]
- 41.Bresson D, von Herrath M. Humanizing animal models: a key to autoimmune diabetes treatment. Sci Transl Med 2011;3:68ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]