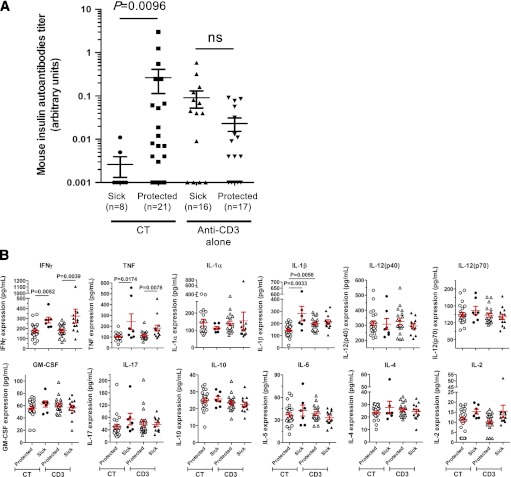
FIG. 1.
Circulating biomarkers predict long-term efficacy of anti-CD3 antibody/oral insulin CT. A: Mouse IAA levels are efficient type 0 biomarkers. After new-onset of T1D, NOD females were randomized to receive anti-CD3 treatment with or without oral insulin. Before treatment randomization, the serum of each individual NOD mouse was collected to measure IAA levels in a blinded fashion. At 15 weeks posttreatment, NOD mice with a BGV <250 mg/dL were considered protected. Data show the results obtained in individual mice (n = 8–21 per group). ns, P > 0.05. B: Serum levels of pro- and anti-inflammatory cytokines in treated NOD mice. Sera from NOD mice treated with anti-CD3 alone or anti-CD3/oral insulin (CT) were collected 2 weeks after treatment started. The concentration of each cytokine was measured by Bioplex assay. At 15 weeks posttreatment, NOD mice with a BGV <250 mg/dL were considered protected. Data show the results obtained in individual mice (n = 7–23 per group). GM-CSF, granulocyte-macrophage colony-stimulating factor.