Abstract
Baculoviruses are one of the most studied insect viruses both in basic virology research and in biotechnology applications. Incorporating an internal ribosome entry site (IRES) into the baculovirus genome generates bi-cistronic baculoviruses expression vectors that produce two genes of interest. The bi-cistronic baculoviruses also facilitate recombinant virus isolation and titer determination when the green fluorescent protein was co-expressed. Furthermore, when the secretion proteins were co-expressed with the cytosolic green fluorescent protein, the cell lysis and cytosolic protein released into the culture medium could be monitored by the green fluorescence, thus facilitating purification of the secreted proteins.
Keywords: baculovirus, bi-cistronic, EGFP, IRES, secretion proteins
Insect cells have been used extensively for the production of recombinant proteins. The relatively low expense of maintenance and ease of scaling up are the primary advantages of using insect cells over their mammalian counterparts. Insect cells can also perform more co-translational and post-translational processes performed by other eukaryotic cells than by commonly used yeast and bacterial expression systems.1-3 Since its discovery in 1983, the insect cell-based baculovirus expression system (BEVS) has been used routinely in industrial laboratories to produce a multitude of diverse types of recombinant proteins for research, medicinal agricultural4 and veterinary applications.5 The baculovirus is an enveloped, double-stranded DNA virus belonging to the Baculoviridae and has been reported to infect over 600 insect species. Among the numerous baculovirus species, Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is the most widely used prototype for basic virology studies and biotechnology applications. The genome of AcMNPV (≈130 kb) is packed into a rod-shaped nucleocapsid, typically 40–50 nm in diameter and 200–400 nm in length.
Baculovirus as a Vector for Recombinant Protein Production
Recombinant AcMNPV-infected insect cells produce more than one thousand recombinant proteins, such as Sf21 or Sf9 derived from Spodoptera frugiperda, or High Five cells derived from Trichoplusia ni. Additionally, recombinant AcMNPV can also infect insect larvae, using the infected larvae as a bioreactor to produce recombinant proteins economically.6 The BEVS approach is based on replacing either the baculovirus’ polyhedrin7 or p10 genes8 with the gene of interest. Both are highly expressed (30% ~50% of the total protein) during the very late stages of infection, and they are non-essential for viral infection and replication in insect cells.9 In the recombinant AcMNPV-infected host cells, the desired genes under the control of polyhedrin or p10 promoters are often expressed in abundance, amounting to approximately 1~500 mg of protein per liter of insect cell culture. Clearly, the most important benefit of using BEVS is the high level production of recombinant proteins during the late phase of viral infection.
Baculovirus as a Vector for Gene Delivery
Although the host range of AcMNPV is the broadest among the baculoviruses, the infection of AcMNPV is nonetheless restricted to insects. However, in the late 1970s and mid-1980s reports showed that mammalian cells internalized baculovirus10-12 and that the virus mediated a very low-level expression of a bacterial gene under the control of polyhedrin or Rous sarcoma virus (RSV) promoters in mammalian cells.13 Two breakthrough papers successfully demonstrated that recombinant AcMNPV can “infect” or transduce mammalian cells. In 1995, Hofmann et al. reported that a recombinant AcMNPV is able to mediate the LacZ gene or luc expression in hepatocytes, provided that the gene expression is driven by the cytomegalovirus (CMV) promoter.14 Subsquently in 1996, Boyce and Bucher depicted efficient baculovirus-mediated expression of the lacZ gene under the control of the RSV promoter in the hepatoma cell line HepG2 and primary rat hepatocytes.15 Although these two pioneering studies suggest that recombinant baculoviruses can only efficiently transduce liver-derived cells, they imply that the recombinant baculoviruses can act as a delivery vehicle for liver-specific genes.15 However, Shoji et al.16 demonstrated that recombinant baculovirus can mediate gene expression in non-hepatic cells, such as HeLa and COS-7 cells, by a chimeric CAG promoter consisting of CMV immediate-early enhancer, chicken β-actin promoter, and rabbit β-globin polyadenylation signal. Since then study, the list of cell lines and primary cells efficiently transduced by baculovirus has significantly expanded16,17 and now includes fish cells.18 Owing to the low cytotoxicity and non-replicative nature of baculovirus in mammalian cells, baculovirus vectors have been employed for in vivo gene delivery.17 Recently, recombinant baculoviruses were employed in mediating recombinant protein production in mammalian cells.19 Thus, baculoviruses can work as vectors for recombinant protein production, both in insect and mammalian cells, or as vehicles for gene delivery. These versatile and useful characteristics make the baculoviruses one of most studied insect viruses.
Bi-Cistronic Baculovirus Expression Vector
In previous studies we demonstrated that it is possible to incorporate an internal ribosome entry site (IRES) into the genome of AcMNPV to mediate bi- or tri-cistronic gene expression in insect cells simultaneously.20-22 We identified two IRESes: the RhPV IRES derived from Rhopalosiphum padi virus20 and PnV539 IRES cloned from Perina nuda picorna-like virus,21 both of which can mediate cap-independent translation in BEVS. These IRESes can be used in the development of bi-cistronic baculovirus expression vectors for the production of heterologous multi-protein complexes. The IRES based bi-cistronic expression vectors have several advantages over the two-promoter-based bi-cistronic vectors. The two-promoter-based bi-cistronic baculovirus transfer vector, like the pFastBacDUAL (a product of Invitrogen), contains the polh and p10 promoters as well as two poly(A) tail signals for simultaneous expression of two genes. Therefore, the size of the two-promoter-based bi-cistronic vector is larger than the IRES-dependent bi-cistronic vector. In addition the greater the amount of the viral late strong promoters integrated into the viral genome, the higher is the transcriptional competition.23
Generating recombinant baculoviruses, requires a homologous recombination reaction. However, homologous recombination between the baculovirus transfer vector and viral DNA is a rare event in insect cells (typically only about 0.1%~1%) and always leads to a background of the wild type virus.24 Homologous recombination requires multiple virus isolation steps to avoid eventual outgrowth of the wild-type virus. Thus, the use of the baculovirus expression vector system is hampered by the slow, tedious procedures for recombinant virus isolation.25 To date, several methods have been developed to resolve this problem. One approach utilizes linearized, essential gene-deleted viral DNA, which cannot initiate viral infection unless rescued by the homologous recombination between the transfer vector that contains the deleted gene and the gene of interest.26 Another approach is the detection of recombinant virus utilizing a reporter gene, β-galactosidase, incorporated into the transfer vector leading to the formation of blue plaques only by recombinant viruses.27 In addition to these in vivo homologous recombination methods, other systems such as direct cloning into the viral genome,28,29 recombination in yeast,30 and transposition in Escherichia coli have also been developed.31 Although all of these approaches may resolve the problems of isolation and purification of the recombinant virus, it is still not easy to determine the titer of the isolated recombinant viruses in experiments, involving recombinant protein production, virus amplification, or gene transduction into mammalian cells. Thus, we employed the RhPV IRES along with the green fluorescent protein EGFP, simultaneously resolving the two major aforementioned bottlenecks in the use of BEVS: virus isolation and titer determination. In recent studies, we showed that the recombinant viruses harboring target genes in the IFNγ32, hemagglutinin,33 neuroligin 1 gene (NL1)34 and the cDNA for the 26S subgenome of the Chikungunya virus (CHIKV 26S RNA)35 can be easily identified under a fluorescent microscope when cloned into the transfer vector (Fig. 1A). All these studies demonstrated that the IRES-EGFP component in the baculovirus transfer vector simplifies recombinant baculoviruses isolation. Because the homologous recombination between the transfer vector and viral DNA is a rare event in insect cells, there is no guarantee that the recombinant virus can be successfully generated for each co-transfection experiment. We found that when fluorescent plaques did not form, the virus progeny were not generated even though there were cells emitting the green fluorescence after the co-transfection experiment. Thus, monitoring virus plaque formation after the co-transfection may be critical for the generation of recombinant viruses. Another practical operation during the preparation of recombinant viruses is to determine the virus titers for the following experiments: recombinant proteins production and preparation of gene delivery vectors. Because the green emission of the EGFP protein as translated by the RhPV 5′UTR IRES is easily identified under a fluorescent microscope, the titer of the first cistronic gene of interest containing the virus can be determined by the simple end-point dilution method.
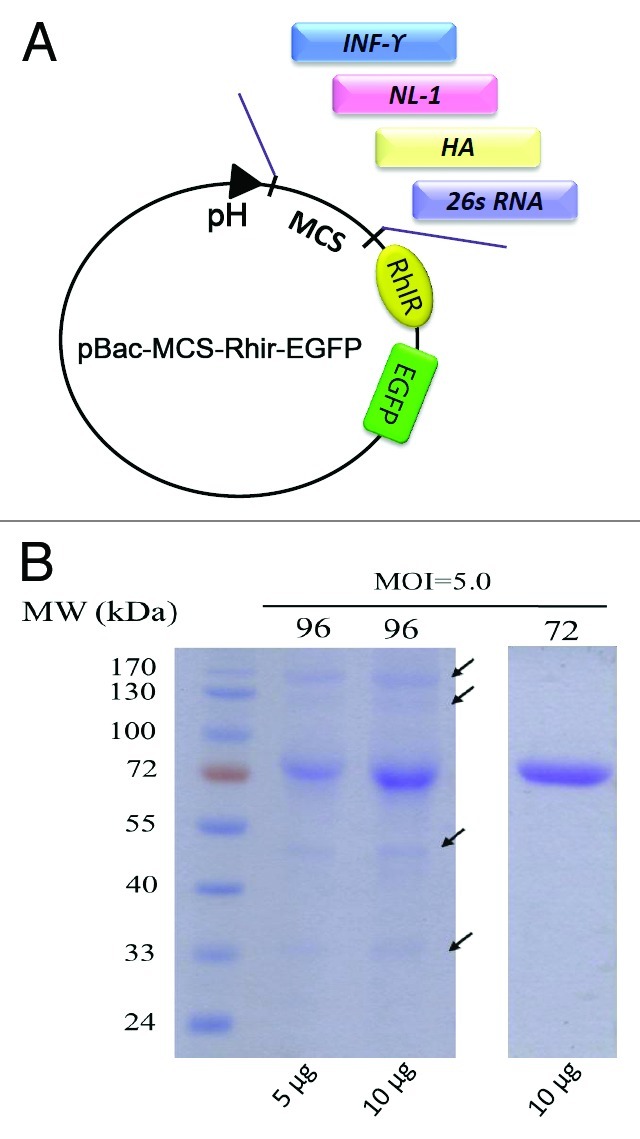
Figure 1. Bi-cistronic baculovirus expression vectors and purification of NL1 proteins from different harvest time. (A) The bi-cistronic baculovirus expression vector, pBac-MCS-Rhir-EGFP. The genes of interest [IFNγ, hemagglutinin, neuroligin 1 genes (NL1) and the cDNA for the 26S subgenome of the Chikungunya virus (CHIKV 26S RNA)] were cloned into the transfer vector. The recombinant virus containing the NL1 gene is named vAcAzSNL1-Rhir-E. pH, the polyhedron promoter; MCS, multiple cloning sites; Rhir, RhPV 5′UTR IRES; EGFP, enhance green fluorescent protein. (B) The Coomassie brilliant blue-staining on SDS-PAGE revealed 3 or 4 visible protein bands (indicated by the arrow), either loading 5 or 10 ug proteins, other than the NL1 protein purify from the vAcAzSNL1-Rhir-E-infected High Five cells harvested at 96 hpi by IMAC. The non-specific bands indicating the presence of contaminants in the medium resulted from cell lysis. In contrast, the NL1 protein purified from the vAcAzSNL1-Rhir-E-infected High Five cells harvested at 72 hpi by IMAC do not contain the contamination proteins even when the 10 ug proteins are loaded into the gel.
Co-Expression of Green Fluorescent Protein to Monitor the Cell Lysis
As we tried to generate the recombinant rat NL1 protein,34 we fused the NL1 gene with signal sequence derived from human Azurocidin gene (AzSP).36 The resulting NL1 protein was secreted into the culture medium. The recombinant virus containing the NL1 gene was named vAcAzSNL1-Rhir-E.34 We found that the optimum harvest time for the NL1 protein before the lysis of infected cells can be determined through the release of cytosolic EGFP. This finding indicated that the RhPV-IRES-EGFP module in the bi-BEVS expression vector—the cytosolic EGFP—can be used to monitor the cell lysis after recombinant virus infection and to signal the release of other intracellular proteins that may interfere with the purification of the NL1 protein. The EGFP (as analyzed by Western blot) was initially found in the culture medium at 96–120 hours post-infection (hpi), and a dramatically increased expression at 144 hpi was observed. These results indicated cell lysis caused by the virus and leaking of the intracellular proteins including cytosolic EGFP into the culture medium beginning 96 hpi. With these results, the culture medium therefore was harvested at 72 hpi avoiding interferences from other intracellular proteins in the succeeding purification. Immobilized metal affinity chromatography (IMAC) was used to purify the recombinant NL1 proteins from the culture medium of High Five cells infected by the recombinant virus. The final purification products were identified using Coomassie brilliant blue-staining on SDS-PAGE, revealing a single band corresponding to the recombinant NL1 protein approximately at 80 kDa with 96% purity (Fig. 1B, lane label 72), determined through quantitative densitometry. We also tried to purify the recombinant NL1 proteins from the vAcAzSNL1-Rhir-E-infected High Five cells harvested at 96 hpi by IMAC. The Coomassie brilliant blue-staining on SDS-PAGE revealed 3 or 4 visible protein bands in addition to the NL1 protein, with only 76% purity as determined by quantitative densitometry (Fig. 1B, the two lanes label 96). These results demonstrated the use of EGFP and bi-BEVS in the straightforward expression of target protein(s) and offset the laborious and tedious multiple chromatographic purification steps. This reflects that the monitoring of EGFP is useful in tracing the lysis of the infected cells and avoiding the interference of other cytosolic proteins during purification. Also, the polyhedrin promoter is a very late promoter, and the expression of EGFP begins at about 1 dpi, suggesting that a copy of egfp gene, present in the host genome or controlled by an earlier promoter, can make the EGFP a more reliable indicator of cell lysis during recombinant viruses infection.
Baculoviruses are promising not only as genetic engineering tools for recombinant protein production but also as an alternative for gene delivery. The incorporation of IRES into the baculovirus’ genome to generate the polycistronic baculoviruses expression vectors will also facilitate the production of hetero-oligomeric proteins and monitor the successful virus infection after the fluorescent reporter gene is co-expressed with the gene of interest.
Acknowledgments
We gratefully acknowledge the financial support of the National Science Council, Taiwan (NSC-98–2321-B-033–001-MY3) to T.Y.W.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/19388
References
- 1.Ailor E, Betenbaugh MJ. Modifying secretion and post-translational processing in insect cells. Curr Opin Biotechnol. 1999;10:142–5. doi: 10.1016/S0958-1669(99)80024-X. [DOI] [PubMed] [Google Scholar]
- 2.Hunt I. From gene to protein: a review of new and enabling technologies for multi-parallel protein expression. Protein Expr Purif. 2005;40:1–22. doi: 10.1016/j.pep.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Yin J, Li G, Ren X, Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol. 2007;127:335–47. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Chen TL, Lin YL, Lee YL, Yang NS, Chan MT. Expression of bioactive human interferon-gamma in transgenic rice cell suspension cultures. Transgenic Res. 2004;13:499–510. doi: 10.1007/s11248-004-2376-8. [DOI] [PubMed] [Google Scholar]
- 5.Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–75. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinn TR, Kao SS, Tseng YC, Chen YJ, Wu TY. Aerosol infectivity of a Baculovirus to Trichoplusia ni larvae: An alternative larval inoculation strategy for recombinant protein production. Biotechnol Prog. 2009;25:384–9. doi: 10.1002/btpr.148. [DOI] [PubMed] [Google Scholar]
- 7.Harrap KA. The structure of nuclear polyhedrosis viruses. I. The inclusion body. Virology. 1972;50:114–23. doi: 10.1016/0042-6822(72)90351-0. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Wilk F, Van Lent JWM, Vlak JM. Immunogold Detection of Polyhedrin, p10 and Virion Antigens in Autographa californica Nuclear Polyhedrosis Virus-infected Spodoptera frugiperda Cells. J Gen Virol. 1987;68:2615–23. doi: 10.1099/0022-1317-68-10-2615. [DOI] [Google Scholar]
- 9.Luckow VA, Summers MD. Trends in the Development of Baculovirus Expression Vectors. Nat Biotechnol. 1988;6:47–55. doi: 10.1038/nbt0188-47. [DOI] [Google Scholar]
- 10.Tjia ST, zu Altenschildesche GM, Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983;125:107–17. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 11.Volkman LE, Goldsmith PA. In vitro study of Autographa californica nuclear polyhedrosis virus interaction with nontarget vertebrate host cells. Appl Environ Microbiol. 1983;45:1085–93. doi: 10.1128/aem.45.3.1085-1093.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granados RR. Replication phenomena of insect viruses in vivo and in vitro In: Safety aspects of baculoviruses as biological pesticides. Miltenburger, HG (Ed. (Bundesministerium fur Forschung und Technologie, Bonn, 1978) [Google Scholar]
- 13.Carbonell LF, Klowden MJ, Miller LK. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985;56:153–60. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci U S A. 1995;92:10099–103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce FM, Bucher NLR. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci U S A. 1996;93:2348–52. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, et al. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol. 1997;78:2657–64. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 17.Hu YC, Yao K, Wu TY. Baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev Vaccines. 2008;7:363–71. doi: 10.1586/14760584.7.3.363. [DOI] [PubMed] [Google Scholar]
- 18.Huang F, Cao S, Cui X, Xiong C, Wang M, Lu Y, et al. Efficient gene delivery into fish cells by an improved recombinant baculovirus. J Virol Methods. 2011;173:294–9. doi: 10.1016/j.jviromet.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Scott MJ, Modha SS, Rhodes AD, Broadway NM, Hardwicke PI, Zhao HJ, et al. Efficient expression of secreted proteases via recombinant BacMam virus. Protein Expr Purif. 2007;52:104–16. doi: 10.1016/j.pep.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Chen YJ, Chen WS, Wu TY. Development of a bi-cistronic baculovirus expression vector by the Rhopalosiphum padi virus 5′ internal ribosome entry site. Biochem Biophys Res Commun. 2005;335:616–23. doi: 10.1016/j.bbrc.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 21.Wu TY, Wu CY, Chen YJ, Chen CY, Wang CH. The 5′ untranslated region of Perina nuda virus (PnV) possesses a strong internal translation activity in baculovirus-infected insect cells. FEBS Lett. 2007;581:3120–6. doi: 10.1016/j.febslet.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 22.Chen WS, Chang YC, Chen YJ, Chen YJ, Teng CY, Wang CH, et al. Development of a prokaryotic-like polycistronic baculovirus expression vector by the linkage of two internal ribosome entry sites. J Virol Methods. 2009;159:152–9. doi: 10.1016/j.jviromet.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Cha HJ, Gotoh T, Bentley WE. Simplification of titer determination for recombinant baculovirus by green fluorescent protein marker. Biotechniques. 1997;23:782–4, 786. doi: 10.2144/97235bm03. [DOI] [PubMed] [Google Scholar]
- 24.Airenne KJ, Peltomaa E, Hytönen VP, Laitinen OH, Ylä-Herttuala S. Improved generation of recombinant baculovirus genomes in Escherichia coli. Nucleic Acids Res. 2003;31:e101. doi: 10.1093/nar/gng102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Chapman DAG, Jones IM. Improving baculovirus recombination. Nucleic Acids Res. 2003;31:E6–6. doi: 10.1093/nar/gng006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitts PA, Possee RD. A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques. 1993;14:810–7. [PubMed] [Google Scholar]
- 27.Kitts PA, Ayres MD, Possee RD. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990;18:5667–72. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst WJ, Grabherr RM, Katinger HW. Direct cloning into the Autographa californica nuclear polyhedrosis virus for generation of recombinant baculoviruses. Nucleic Acids Res. 1994;22:2855–6. doi: 10.1093/nar/22.14.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gritsun TS, Mikhailov MV, Roy P, Gould EA. A new, rapid and simple procedure for direct cloning of PCR products into baculoviruses. Nucleic Acids Res. 1997;25:1864–5. doi: 10.1093/nar/25.9.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel G, Nasmyth K, Jones N. A new method for the isolation of recombinant baculovirus. Nucleic Acids Res. 1992;20:97–104. doi: 10.1093/nar/20.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67:4566–79. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen WS, Villaflores OB, Jinn TR, Chan MT, Chang YC, Wu TY. Expression of recombinant human interferon-γ with antiviral activity in the bi-cistronic baculovirus-insect/larval system. Biosci Biotechnol Biochem. 2011;75:1342–8. doi: 10.1271/bbb.110107. [DOI] [PubMed] [Google Scholar]
- 33.Jinn TR, Khac NT, Wu TY. Production of H5N1 hemagglutinin in Trichoplusia ni larvae by a novel bi-cistronic baculovirus expression vector. Proceedings of World Academy of Science. Engineering and Technology. 2010;65:984–7. [Google Scholar]
- 34.Chen WS, Villaflores OB, Lu CF, Wu HI, Chen YJ, Teng CY, et al. Functional expression of rat neuroligin-1 extracellular fragment by a bi-cistronic baculovirus expression vector. Protein Expr Purif. 2012;81:18–24. doi: 10.1016/j.pep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Kuo SC, Chen YJ, Wang YM, Kuo MD, Jinn TR, Chen WS, et al. Cell-based analysis of Chikungunya virus membrane fusion using baculovirus-expression vectors. J Virol Methods. 2011;175:206–15. doi: 10.1016/j.jviromet.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Olczak M, Olczak T. Comparison of different signal peptides for protein secretion in nonlytic insect cell system. Anal Biochem. 2006;359:45–53. doi: 10.1016/j.ab.2006.09.003. [DOI] [PubMed] [Google Scholar]


