Abstract
Plant response to water deficit and subsequent re-watering is fine tuned at the whole plant level. It differs not only between shoot and root, but also among particular leaves along a plant axis. We estimated the expression of proline metabolism-related genes and the activity of senescence-related promoter in roots and individual leaves of tobacco plants in the course of drought stress and recovery. Proline plays the dual role of an osmoprotectant and an antioxidant under water deficit. High proline concentration in the youngest uppermost leaves contributed to their protection from drought, which was associated with low degree of senescence. During recovery, elevated proline concentrations persisted and the senescence-related promoter was switched off in all surviving leaves. Two mutually exclusive scenarios were followed by tobacco leaves on recovery—restoration of photosynthesis and metabolism, or death, depending on the progress of senescence.
Unlike animals, plants cannot respond to adverse environmental conditions by running away; they have to survive on the spot. During their lifetime, they may have to cope with a number of biotic and abiotic stress factors, of which one of the most frequent and severe is water deficit. Long-term water shortage may threaten agricultural production over large geographic areas. Understanding the mechanisms by which plants respond to drought and by which they recover from it is crucially important, particularly in the light of recent global climatic changes. Plants respond to drought by a plethora of reactions, including fast changes, such as stomatal closure to reduce their rate of water loss by transpiration, as well as a substantial modulation of their metabolism and growth.1,2 Drought-induced cessation of shoot growth is accompanied by the inhibition of new leaf initiation and by the accelerated senescence of older leaves. The decrease in canopy area ensures lower transpiration and higher water retention.3 In contrast to shoots, roots continue to grow, albeit with some morphological modifications, in order to reach water in deeper layers—primary roots elongate, but branching is diminished.4-6 A balance between growth and carbon supply is achieved through a complex regulatory network in which sugars (e.g., glucose, sucrose and starch) and phytohormones, mainly ABA (abscisic acid) and cytokinins (CK) perform central roles.6-9
The stomatal closure caused by water deficit not only reduces transpiration, but lowers gas exchange generally. This result in decreased CO2 diffusion to the chloroplasts, which downregulates carbon assimilation. NADPH consumption by the Calvin cycle decreases, causing an increase in NADPH:NADP+ ratio. Over reduction of components within the electron-transport chain in the chloroplast thylakoid membranes cannot be balanced by availability of the electron acceptor NADP+. Instead, electrons are captured by water resulting in the generation of ROS (reactive oxygen species).10 Under conditions of reduced rates of photosynthesis, the excess reducing power needs to be further diminished by photoinhibition and/or by an increase in photorespiration.11 Under water stress, metabolic pathways which convert excess NADPH into NADP+ are also stimulated.
An example of such a pathway is the elevation of proline biosynthesis from glutamic acid in the chloroplasts of water-stressed plants, which consumes NADPH, decreases the NADPH:NADP+ ratio in chloroplasts and in turn reduces ROS production.12 Thus, proline functions not only as an osmoprotectant, a molecular chaperone, a pH buffer and a source of carbon and nitrogen during recovery, but also as important compound decreasing ROS concentrations in both direct and indirect ways.13
To understand proline metabolism at a whole plant level, we measured free proline concentrations in leaves and roots of tobacco under drought stress and during subsequent recovery.14 We also determined transcript abundances of three copies of the PDH gene encoding proline dehydrogenase (PDH), the key enzyme in proline degradation, and of two P5CS genes coding for the rate-limiting proline biosynthetic enzyme, Δ1-pyrroline-5-carboxylate synthetase.15 Consistent with other studies, PDH genes were downregulated and P5CS genes upregulated under prolonged drought stress in tobacco.16,17 However, the stress response differed markedly between root and shoot. The P5CS A gene, which responded to drought more intensely than the P5CS B gene, was upregulated to a higher level in the shoot than in the root. Its transcript level was also much higher in the three uppermost leaves than in middle and bottom ones. Free proline concentrations correlated well with levels of expression of the proline metabolism genes, being highest in the upper leaves. Photosynthesizing tissues, mainly the youngest and the most metabolically active leaves, also maintained the high free proline concentrations. Our results were consistent with the suggested roles for proline as an ROS scavenger and of proline biosynthesis as metabolic process that diminishes an excess of reducing power in chloroplasts of plants under drought.12,18 The antioxidant function of proline and its biosynthesis in chloroplasts seems to be at least as essential for drought survival as its role as an osmoprotectant.
Proline also seems to play an important role during recovery from drought stress. While rehydration is quickly perceived by the plant, which is reflected for example in the fast downregulation of expression of dehydrin encoding genes, the P5CS A transcript level and free proline content remain high in tobacco leaves. As dehydrins were reported to change membrane composition and concomitantly photosynthetic capacity, their high content might be unfavorable under well-watered conditions.19 In contrast with dehydrins, the levels of both proline biosynthesis-related transcripts and free proline descended, but remained well above the basal level for at least some time after the stress relief. Interestingly, the uppermost leaves with the highest proline concentration also showed the highest upregulation of the CSP41a gene, which is involved in chloroplast mRNA turnover. This observation emphasized the importance of proline for the restoration of chloroplast function on recovery from drought.
When water shortage is relieved, the plants need to restart growth as quickly as possible and this requires modulation of the normal senescence program. In order to follow senescence under drought stress and subsequent recovery in more detail, we employed SAG12:ZOG1 transgenic tobacco plants.6 The expression of ZOG1 can be used as a marker of the activity of senescence related promoter SAG12.20 This gene encodes a trans-zeatin O-glucosyltransferase, an enzyme which converts the physiologically highly active cytokinin trans-zeatin into its storage form (trans-zeatin O-glucoside).21 SAG12:ZOG1 transgenic plants exhibited elevated total CK content, but the level of bioactive CKs was not significantly different from the corresponding wild-type. The transgenic plants exhibited a mild delay in the senescence of drought-stressed lower leaves and a little faster recovery. However, their drought stress response was comparable to that of wild type plants.6,22 We preferred the analysis of the SAG12:ZOG1 construct to endogenous senescence-controlled genes because ZOG1 expression was driven exclusively by the SAG12 promoter and not modulated by additional regulatory motifs. As the differences in the expression of proline metabolic genes did not reach statistical significance between the studied genotypes, we used this transformant to evaluate the expression of stress related genes.
As demonstrated in Table 1, SAG12 promoter activity was strongly stimulated during prolonged drought stress. Nevertheless, the gradient between young and old leaves was maintained, only its steepness being enhanced. After re-watering, SAG12 activity was strongly inhibited. Promoter activity was much lower in re-watered plants than in the corresponding well-watered controls, which already exhibited natural senescence (Fig. 1). However, the lower yellow drought-stressed leaves, which exhibited the highest SAG activity, died. It seems that there is a threshold in the process of leaf senescence, beyond which reversal to an active metabolic state is no longer possible. Leaves seem to follow two mutually exclusive scenarios on recovery—restoration of photosynthesis and metabolism, or death. The decision between the two fates depends on the degree of senescence in a particular leaf.
Table 1. The activity of the senescence-associated promoter SAG12 during the drought stress and subsequent recovery.
| Leaf number |
6-d drought |
1-d recovery |
Control |
|||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| 1 |
0 |
0 |
0 |
0 |
0 |
0 |
| 2 |
0 |
0 |
0 |
0 |
0 |
0 |
| 3 |
0,001 |
0,001 |
0,005 |
0,005 |
0 |
0 |
| 4 |
0,010 |
0,004 |
0,001 |
0,001 |
0,003 |
0,001 |
| 5 |
0,036 |
0,004 |
0,001 |
0 |
0,018 |
0,004 |
|
6* |
0,535* |
0,012 |
0,017 |
0,002 |
0,300* |
0,042 |
| 7 |
0,367 |
0,010 |
0,130 |
0,047 |
1,200 |
0,250 |
| 8 |
9,109 |
0,406 |
|
|
1,653 |
0,312 |
| 9 |
|
|
|
|
4,351 |
0,330 |
| 10 |
|
|
|
|
14,160 |
1,106 |
| 11 |
|
|
|
|
15,994 |
0,659 |
| 12 | 18,417 | 1,280 | ||||
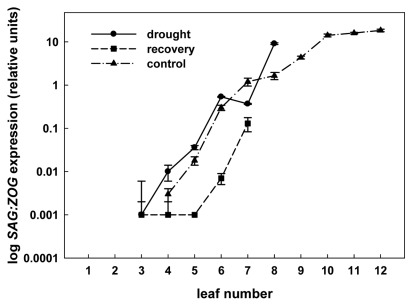
The relative ZOG transcript levels (normalized against Act9 according to Dobr´ et al. 2011) in SAG12:ZOG1 transgenic tobacco leaves along the axis, numbered from the top.Dramatic increase in ZOG expression, which reflected senescence-related SAG12 promoter activity, was observed in the sixth leaf in drought-stressed and control plants (marked by asterisk). SAG12 activity remained low in all leaves during recovery. SE –, standard error.
Figure 1. The relative ZOG transcript levels in transgenic SAG12:ZOG1 tobacco leaves along the axis, numbered from the top and expressed in logarithmic scale. Low ZOG expression and thus low activity of a senescence-related SAG12 promoter was measured in all surviving leaves during recovery. Three plants were analyzed under each experimental treatment, transcript levels were measured in two independent qRT PCR assays.
Thus, recovery after stress is a very complex process involving rearrangements of many metabolic pathways. It is not just a return to the state before stress initiation.12,23 The young and middle leaves, protected during the drought stress, rapidly increase their rate of photosynthesis.21,24 This results in enhanced carbon assimilation enabling the re-establishment of plant growth. However, the oldest leaves, in which senescence is most advanced, are lost. Thus, canopy area is further reduced on drought recovery, but surviving leaves show higher metabolic activity than leaves of control plants which were not exposed to stress.3,8,23
Our results document that both stress response and recovery are fine tuned at the whole plant level. Whereas the plant rejuvenates some parts, others are sacrificed depending on the degree of their senescence.
Acknowledgment
We thank to Professor David Morris for a critical reading of this manuscript and Dr. Alena Gaudinov´ for drawing the figure. This work was supported by the Grant Agency of the Czech Republic, project No. 206/09/2062 and 522/09/2058 and by the Ministry of Education,Youth and Sports CR, no. NPVII 2B08058.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18375
References
- 1.Novikova GV, Moshkov IE, Los DA. Protein sensors and transducers of cold and osmotic stress in cyanobacteria and plants. J Mol Biol. 2007;41:427–37. doi: 10.1134/S0026893307030089. [DOI] [PubMed] [Google Scholar]
- 2.Hayano-Kanashiro C, Calderon-Vazquez C, Ibarra-Laclette E, Herrera-Estrella L, Simpson J. Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE. 2009;4:e7531. doi: 10.1371/journal.pone.0007531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira JS, Chaves MM. Plant water deficits in Mediterranean ecosystems. In: Smith JAC, Griffiths H, eds. Plant responses to water deficits – from cell to community. Oxford: BIOS Scientific 1993; 237-51. [Google Scholar]
- 4.Sharp RE. Interaction with ethylene: changing views on the role of abscisic acid in root and shoot responses to water stress. Plant Cell Environ. 2002;25:211–22. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 5.Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot. 2006;97:883–93. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havlov´ M, Dobrev PI, Motyka V, Štorchov´ H, Libus J, Dobr´ J, et al. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008;31:341–53. doi: 10.1111/j.1365-3040.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 7.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signalling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 8.Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to drought and cold stress. Curr Opin Biotechnol. 1996;7:161–7. doi: 10.1016/S0958-1669(96)80007-3. [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Tom´s Werner T, et al. Tran. LSP Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and ABA responses, and ABA biosynthesis. Plant Cell. 2011;23:2169–2183. doi: 10.1105/tpc.111.087395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro C, Chaves MM. Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot. 2011;62:869–82. doi: 10.1093/jxb/erq340. [DOI] [PubMed] [Google Scholar]
- 11.Rivero RM, Shulaev V, Blumwald E. Cytokinin-dependent photorespiration and the protection of photosynhtesis during water deficit. Plant Physiol. 2009;150:1530–40. doi: 10.1104/pp.109.139378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di´az P, Betti M, Sanchez DH, Udvardi MK, Monza J, Marquez AJ. Deficiency in plastidic glutamine synthetase alters proline metabolism and transcriptomic response in Lotus japonicus under drought stress. New Phytol. 2010;188:1001–13. doi: 10.1111/j.1469-8137.2010.03440.x. [DOI] [PubMed] [Google Scholar]
- 13.Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Dobr´ J, Motyka V, Dobrev P, Malbeck J, Pr´šil IT, Haisel D, et al. Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J Plant Physiol. 2010;167:1360–70. doi: 10.1016/j.jplph.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Dobr´ J, Vankov´ R, Havlov´ M, Burman AJ, Libus J, Štorchov´ H. Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. J Plant Physiol. 2011;168:1588–97. doi: 10.1016/j.jplph.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell. 1996;8:1323–35. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sze´kely G, Abraham E, Cselo A, Rigo G, Zsigmond L, Csiszar J, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- 18.Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–9. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- 19.Beck JG, Mathieu D, Loudet C, Buchoux S, Dufourc EJ. Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J. 2007;21:1714–23. doi: 10.1096/fj.06-7809com. [DOI] [PubMed] [Google Scholar]
- 20.Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–8. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 21.Martin RC, Mok MC, Mok DWS. Isolation of a cytokinin gene, ZOG1, encoding zeatin O-glucosyltransferase from Phaseolus lunatus. Proc Natl Acad Sci USA. 1999;96:284–9. doi: 10.1073/pnas.96.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haisel D, Vaňkov´ R, Synkov´ H, Pospi´šilov´ J. The impact of trans-zeatin O-glucosyltransferase gene over-expression in tobacco on pigment content and gas exchange. Biol Plant. 2008;52:49–58. doi: 10.1007/s10535-008-0007-6. [DOI] [Google Scholar]
- 23.Oono Y, Seki M, Nanjo T, Narusaka M, Fujita M, Satoh R, et al. Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using ca. 7000 full-length cDNA microarray. Plant J. 2003;34:868–87. doi: 10.1046/j.1365-313X.2003.01774.x. [DOI] [PubMed] [Google Scholar]
- 24.Chaves MM. Effects of water deficits on carbon assimilation. J Exp Bot. 1991;42:1–16. doi: 10.1093/jxb/42.1.1. [DOI] [Google Scholar]