Abstract
GC, GC–MS, and HPLC–LLSD analyses were used to identify and quantify cuticular and internal lipids in males and females of the blow-fly (Lucilia sericata). Sixteen free fatty acids, seven alcohols and cholesterol were identified and quantitatively determined in the cuticular lipids of L. sericata. Cuticular fatty acids ranged from C6 to C20 and included unsaturated entities such as 16:1n-9, 18:1n-9, 20:4n-3 and 20:5n-3. Cuticular alcohols (only saturated and even-numbered) ranged from C12 to C20 in males and C10 to C22 in females. Only one sterol was found in the cuticular lipids of both males and females. 23 free fatty acids, five alcohols and cholesterol were identified in the internal lipids. Internal fatty acids were present in large amounts—7.4 mg/g (female) and 10.1 mg/g (male). Only traces of internal alcohols (from C14 to C26 in males, from C14 to C22 in females) were found in L. sericata. Large amounts of internal cholesterol were identified in L. sericata males and females (0.49 and 0.97 mg/g of the insect body, respectively).
Keywords: Cuticular and internal lipids, HPLC–LLSD, GC–MS, Lucilia sericata, Conidiobolus coronatus, Fungal infection
Introduction
Lucilia species (Calliphoridae), important pollinators of flowering plants, are distributed worldwide and are the best known species in human infestation in America, Africa, and Asia. These ectoparasites are found in the meat and corpses of animals, and cause myiasis in humans and domestic herbivorous animals [1–3]. In 1826, myiasis caused by L. sericata in humans was reported by Magen. It was then that the parasites were isolated from the mouth, eyes, and paranasal sinuses of a hospital patient for the first time.
Unlike the larvae of some other myiasis-causing flies, L. sericata larvae rarely invade living healthy tissues surrounding a necrotic wound. Due to this fascinating phenomenon, called facultative myiasis, L. sericata larvae have been used since antiquity as a safe and effective wound treatment. The secretions of maggots are known to stimulate in vitro increase in total human fibroblasts [4] and have antibacterial properties [5]. Although no in vivo reports regarding wound healing mechanisms of maggot therapy are available, recent data show that fly cuticular fatty acids may play a significant role in this process. Fatty acid extracts of dried L. sericata larvae called in traditional Chinese medicine “WuGuChong” and used to treat superficial purulent diseases such as furuncle or carbuncle, can promote murine cutaneous wound healing probably resulting from the powerful angiogenic activity of the extracts [6].
The cuticle of all insects is covered with a very thin epicuticular layer of wax. The cuticle consists of several layers, from the outside to the inside: the epicuticle, the procuticle and the epidermis. The insect cuticle is the first barrier against biological or chemical contact insecticides; it is mostly resistant to enzyme degradation and exhibits characteristic water barrier properties [7].
Naturally occurring entomopathogens are important regulatory factors of insect populations. The potential use of fungal pathogens to control insects is well documented, and the use of fungi as control agents against insect pests has been reviewed [8–12]. Susceptibility or resistance of various insect species to fungal invasion may result from several factors, including differences in the structure and composition of the exoskeleton, the presence of antifungal compounds in the cuticle, as well as the efficiency of cellular and humoral defense reactions of the invaded insect [13]. It is believed that the epicuticular lipid profile of the insect host may be one of pivotal factors determining insect susceptibilities or resistance to fungal attack [14]. The mode of action of entomopathogenic fungi involves the attachment of fungal spores to the cuticle, followed by spore germination and, depending on the fungal species, formation of appressorium or penetrative hyphae. Penetration is then initiated, involving both mechanical and enzymatic mechanisms [15]. Once inside the host, the fungus propagates, consuming nutrients and releases metabolites (some of which might be toxic), which results in mycosis and, ultimately, host death [9, 10].
The ability of the fungus to fully degrade the epicuticular hydrocarbon components of its insect host, utilizing them as an exogenous carbon source, was demonstrated by Napolitano and Juarez [16]. The presence of a wax layer potentially affects spore germination by fungilytic or fungistatic toxicity, or by acting as a barrier to the chitin matrix of the insect exoskeleton, effectively preventing the spore from coming into contact with nutrients or other cues that trigger germination. Thus, the waxy layer produced on the cuticle may act as a first line of defense against fungal pathogens. Little information on both, stimulatory and inhibitory effects of cuticular fatty acids on growth and virulence of insecticidal fungi is available. Medium- and short-chain fatty acids and alcohols have been demonstrated to be toxic to filamentous fungi, including some that have been isolated specifically from insect cuticle [17, 18], but the toxicity of long-chain fatty acids is unknown. Kerwin [19] demonstrated the toxic effects of 6:0, 7:0, 9:0, 10:0, 18:2 and 18:3 fatty acids on Entomophthora culicis conidia. However, 18:1 was found to have a positive effect on spore germination and to mitigate the harmful effects of 18:3 acid. The concentration of fatty acids also had an important impact on fungal growth and conidia development: 0.1% 16:1 was toxic to secondary conidia, but positively affected fungal growth. Cuticular lipids were found to have toxic or inhibitory effects on the conidia of B. bassiana and P. fumosoroseus when the spores were germinated on nutrient agar in the presence of lipids [19]. Eighteen fatty acids identified in the larval cuticle of three insect species representing differing susceptibilities to Conidiobolus coronatus infection [20], were thoroughly tested for effects on the in vitro growth and pathogenicity of this parasitic fungus [21].
This paper describes the cuticular lipid composition of L. sericata adults. The surface lipids of flies were separated into classes of compounds using HPLC–LLSD. Qualitative and quantitative analyses were done by GC and GC–MS. The determination of the composition of fly lipids and their impact on the development and pathogenicity of C. coronatus may have great practical importance and will allow using this fungus or its metabolites to control insect populations.
Materials and Methods
Insects
Lucilia sericata raised from eggs laid on beef by adult flies, were reared at 25 °C with 50% relative humidity and a 12:12 h photoperiod. Maternal generation was maintained in the same conditions. The insects were fed on beef and it took them approximately 7 days from hatching to puparium formation and another 7 days to the appearance of adult. The insects were exposed for 18 h to fully grown and sporulating fungal colonies. Ten flies were kept in each Petri dish (males and females separately). The adults exposed for 18 h to sterile uninoculated Sabouraud agar supplemented with G. mellonella larval extract served as a control. After exposure, the insects were transferred to clean Petri dishes with sugar and water and kept under their growing conditions for 10 days. The condition of the exposed animals was monitored daily. Exposure of tested insects to a C. coronatus colony for 18 h was found to be most efficient method resembling the natural infection process [22]. In order to avoid pseudo replication, all assays of fungi vs. insects were performed with the use of flies from different stocks incubated in three different chambers.
A culture of the wax moth, Galleria mellonella was maintained and reared in temperature and humidity controlled chambers (30 °C, 70% r.h.) in constant darkness on an artificial diet [23]. Fully grown larvae were collected before pupation, surface sterilized, homogenized and used as a supplement in fungal cultures.
Fungus
Conidiobolus coronatus, isolate number 3491, originally isolated from Dendrolaelaps spp., was obtained from the collection of Prof. Bałazy (Polish Academy of Sciences, Research Center for Agricultural and Forest Environment, Poznań), routinely maintained in 90-mm Petri dishes at 20 °C with cyclic changes of light (L:D 12:12) on Sabouraud agar medium (SAM) with the addition of homogenized Galleria mellonella larvae to a final concentration of 10% wet weight. Addition of homogenized G. mellonella larvae enhances SAM cultures of C. coronatus. The levels of mycelial growth, conidia production, and virulence were good in hundreds of successive transfers [24] suggesting a stimulatory effect of insect proteins, carbohydrates and lipids on C. coronatus growth and insecticidal properties.
Extraction of Insects
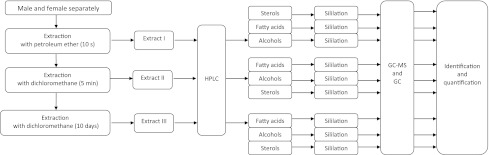
Figure 1 shows the scheme for preparing the sample and analysis. Male and female specimens of L. sericata were extracted first in petroleum ether for 10 s and then a second time in dichloromethane for 5 min [20]. These two extracts (petroleum extract I and dichloromethane extract II) contained cuticular lipids. The third extraction was a long one with dichloromethane for 10 days (III extract). This third extract contained internal lipids. Then, 0.5 ml of the whole extract was placed in a glass flask and then evaporated under nitrogen to determine the dry mass of the remaining extracted lipids. Table 1 lists the number of adult insects, as well as the masses of the extracts.
Fig. 1.
Scheme of the analysis
Table 1.
Quantitative summary of the experiment: numbers and masses of insect; masses of lipids
| Number of insects | Insects (g) | Extracts | Lipids in extracts (mg) | Lipids | ||
|---|---|---|---|---|---|---|
| (mg/insect) | (mg/g of the insect body) | |||||
| Male | 75 | 1.7 | I | 5.1 | 0.07 | 3.0 |
| II | 1.3 | 0.02 | 0.8 | |||
| III | 42.3 | 0.56 | 24.9 | |||
| Female | 80 | 2.3 | I | 3.2 | 0.04 | 1.4 |
| II | 1.6 | 0.02 | 0.7 | |||
| III | 45.4 | 0.57 | 19.7 | |||
I petroleum extract (10 s)
II dichloromethane extract (5 min)
III dichloromethane extract (10 days)
High Performance Liquid Chromatography
All lipid extracts of the males and females (I, II and III) were separated into several classes of compounds by HPLC in the normal phase using a Shimadzu LP-6A binary pump in gradient mode. A laser light scattering detector was used as the detection system [25]. To obtain large amounts of lipids for GC–MS analysis the separation was repeated five times. All fractions were evaporated, silylated and analyzed by GC and GC–MS.
Gas Chromatography
The investigations were carried out on a Clarus 500 (Perkin Elmer) gas chromatograph equipped with a Rtx 5 fused silica column (30 m × 0.25 mm i.d., film thickness 0.1 μm) was used. The oven temperature was held at 80 °C for 10 min and then increased at a rate of 4 °C/min to a final temperature of 320 °C [25].
Gas Chromatography–Mass Spectrometry
Gas chromatography–mass spectrometry measurements were carried out by coupling an SSQ 710 (Finnigan Mat) spectrometer to a Hewlett-Packard 5890 gas chromatograph. The samples were introduced through the gas chromatograph equipped with a 30 m × 0.25 mm i.d., Optima-5 silica capillary column and with a 0.25 μm thick film. The oven temperature 80 °C (held for 10 min) was increased to 320 °C at 4 °C/min. The injector temperature was 320 °C and the carrier gas was helium. The ion source was maintained at 220 °C.
The lipids were derivatized by methods described previously [20]. All compounds were identified by comparing the retention time of the analyzed compounds with standards and on the basis of silyl derivative ions. The mass spectrum of trimethylsilyl ethers of fatty acids showed the following ions: M+· (molecular ion), [M−15]+, and fragment ions at m/z 117, 129, 132, and 145. Cholesterol was identified on the basis of the characteristic ions of silyl derivatives (m/z 458 (M+·), 129, 329, 368, 145, 121 and 353). Characteristic ions of alcohols (trimethylsilyl derivatives) were [M−15]+ and m/z 103 [26].
Statistical Analysis
In order to quantitatively determine each of the compounds analyzed, GC analysis was performed with internal standards (19-methylarachidic acid, 1-octacosanol and sitosterol). Data are presented as the means ± standard deviations of three separate analyses performed on different samples. The data obtained were statistically processed by using the t pairwise test for determining significant differences in males and females lipids concentration.
Results
Susceptibility of L. sericata to Fungal Infection
Exposure of L. sericata adults to the sporulating C. coronatus resulted in the prompt death of all tested males and females (Table 2). Both, males and females died around the termination of the 18-h exposure to the fungal culture. The lack of resistance of L. sericata males and females on C. coronatus infection are confirmed by results obtained with the t pairwise test. Based on the t pairwise test we did not notice any significant differences in males and females susceptibility to fungal infection.
Table 2.
The susceptibility of Lucilia sericata males and females to fungal infection
| Developmental stage/treatment | Number of insects | Mortality (%)a |
|---|---|---|
| Adult females | ||
| Control | 30 | 0 |
| Exposed to C. coronatus | 50 | 100 |
| Adult males | ||
| Control | 47 | 0 |
| Exposed to C. coronatus | 50 | 100 |
aAdult insects were exposed to sporulating C. coronatus colonies as described in the "Materials and Methods" section. The insect susceptibility to fungal infection is expressed as percentage of mortality in tested populations
Extraction of L. sericata Lipids
This is the first time that the chemical composition of the cuticular and external lipids in the L. sericata has been analysed and identified. Three extractions of male and female L. sericata were performed. The petroleum ether (I) and dichloromethane (II) extracts of males yielded 3.0 and 0.8 mg/g of the insect body, respectively. These two extracts consisted of cuticular lipids, so the quantity of lipids amounted to 3.8 mg/g of the insect body. The quantities of male internal lipids yielded 24.9 mg/g of the insect body (0.56 mg/insect). The quantities of cuticular lipid extracts obtained from female L. sericata were smaller than those from the males and amounted 2.1 mg/g of the insect body (1.4 and 0.7 mg/g of the insect body in extracts I and II, respectively). Also, the quantities of female internal lipids were less than in the males and yielded 19.7 mg/g of the insect body, but the quantity of internal lipids per insect was almost the same (0.57 mg/female vs. 0.56 mg/male).
Extracts of lipids from male and female L. sericata were separated by HPLC–LLSD into fractions containing general groups of chemical entities. The lipids extracted from the males and females contained four fractions: hydrocarbons, triacylglycerols, free fatty acids (FFA) and sterols. The triacylglycerols were present in the internal lipids of male and female (III extracts). They serve as a source of energy stored in fat body. The hydrocarbons were present in cuticular lipids (I and II extracts) in small amounts. They can play an important role as pheromones. In this study were determined compounds with potential antimicrobial activity, including the free fatty acid, alcohol and sterol fractions. These fractions were further analyzed by GC and GC–MS. For qualitative purposes, the instrument was operated in total ion current (TIC) mode and single ion monitoring (SIM) mode for monitoring [M−15]+ ions (Table 4). Single ion monitoring mode was used to achieve high selectivity and sensitivity. Gas chromatography was used for the quantitative analyses of lipids. Comparisons were made with the lipids extracted from male and female L. sericata.
Table 4.
Chemical composition of the internal fatty acids (extract III) found in males and females of Lucilia sericata
| FFA | Content (μg/1 g) | Relative content % (w/w) | [M−15]+ Monitored ions (SIM mode) | ||
|---|---|---|---|---|---|
| Internal FFA (male) | Internal FFA (female) | Internal FFA (male) | Internal FFA (female) | ||
| 6:0 | 1.8 ± 0.1 | 1.33 ± 0.05 | <0.1 | <0.1 | 173 |
| 7:0 | – | 0.40 ± 0.02 | – | <0.1 | 187 |
| 8:0 | 0.86 ± 0.04 | 0.96 ± 0.05 | <0.1 | <0.1 | 201 |
| 9:0 | 0.59 ± 0.03 | 0.40 ± 0.02 | <0.1 | <0.1 | 215 |
| 10:0 | – | Traces | – | Traces | 229 |
| 11:0 | – | – | – | – | 243 |
| 12:0 | 10.4 ± 0.8 | 11.0 ± 0.5 | 0.1 | 0.1 | 257 |
| 14:1n-9 | – | Traces | – | Traces | 283 |
| 14:0 | 16.4 ± 0.9 | 13.1 ± 0.5 | 0.2 | 0.2 | 285 |
| 15:0 | 1.9 ± 0.1 | 2.0 ± 0.1 | <0.1 | <0.1 | 299 |
| 16:1n-9 | 14.8 × 102 ± 0.5 × 102 | 12.9 × 102 ± 0.3 × 102 | 14.7 | 17.5 | 311 |
| 16:0 | 19.7 × 102 ± 0.6 × 102 | 15.5 × 102 ± 0.5 × 102 | 19.5 | 21.0 | 313 |
| 17:1n-10 | 6.1 × 101 ± 0.3 × 101 | 3.9 × 101 ± 0.2 × 101 | 0.6 | 0.5 | 325 |
| 17:0 | 8.2 ± 0.4 | 6.6 ± 0.5 | 0.1 | 0.1 | 327 |
| 18:2n-6 | 2.6 × 101 ± 0.1 × 101 | 14.7 ± 0.6 | 0.3 | 0.2 | 337 |
| 18:1n-9 | 5.8 × 103 ± 0.2 × 103 | 39.3 × 102 ± 1 × 102 | 57.4 | 53.2 | 339 |
| 18:0 | 22.8 × 101 ± 0.9 × 101 | 16.5 × 101 ± 0.7 × 101 | 2.3 | 2.2 | 341 |
| 19:0 | Traces | Traces | Traces | Traces | 355 |
| 20:5n-3 | 4.2 × 102 ± 0.1 × 102 | 29.3 × 101 ± 0.7 × 101 | 4.2 | 4.0 | 359 |
| 20:4n-3 | 13.8 × 101 ± 0.4 × 101 | 8.9 × 101 ± 0.3 × 101 | 1.4 | 1.2 | 361 |
| 20:1n-6 | Traces | 1.23 ± 0.08 | Traces | <0.1 | 367 |
| 20:0 | 6.5 ± 0.3 | 4.6 ± 0.2 | 0.1 | 0.1 | 369 |
| 22:0 | 1.59 ± 0.06 | 0.97 ± 0.06 | <0.1 | <0.1 | 397 |
| 24:0 | 4.7 ± 0.2 | 3.0 ± 0.2 | <0.1 | <0.1 | 425 |
| 26:0 | 0.81 ± 0.03 | 0.61 ± 0.03 | <0.1 | <0.1 | 453 |
| Sum | 10.1 × 103 | 73.9 × 102 | – | – | – |
Data are presented as the means ± standard deviations of three separate analyses performed on different samples
Fatty Acid Composition of Male L. sericata
Table 3 summarizes the results obtained during the identification and quantification of FFA in the cuticular lipids of male L. sericata. The two cuticular extracts (petroleum ether-I and dichloromethane-II) contained 15 compounds from C6 to C20. The dominant cuticular fatty acids (all with an even number of carbon atoms) were: 16:1n-9 (10.3%), 16:0 (29.4%), 18:1n-9 (40.6%) and 18:0 (6.7%). The total free fatty acid content in cuticles in males was 14.3 μg/g (6.1 μg/g in the petroleum ether extract and 8.2 μg/g in the dichloromethane extract).
Table 3.
Chemical composition of the cuticular fatty acids found in males and females of Lucilia sericata
| FFA | Content (μg/1 g) | Relative content % (w/w) | ||
|---|---|---|---|---|
| Cuticular FFA (male) | Cuticular FFA (female) | Cuticular FFA (male) | Cuticular FFA (female) | |
| 6:0 | 0.47 ± 0.04 | 0.14 ± 0.02 | 3.3 | 1.6 |
| 7:0 | – | 0.15 ± 0.01 | – | 1.7 |
| 8:0 | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.8 | 1.4 |
| 9:0 | 0.45 ± 0.03 | 0.32 ± 0.04 | 3.1 | 3.6 |
| 10:0 | 0.14 ± 0.02 | 0.14 ± 0.01 | 1.0 | 1.6 |
| 11:0 | Traces | Traces | Traces | Traces |
| 12:0 | 0.26 ± 0.03 | 0.28 ± 0.03 | 1.8 | 3.2 |
| 14:1n-9 | – | – | – | – |
| 14:0 | 0.31 ± 0.03 | 0.31 ± 0.03 | 2.2 | 3.5 |
| 15:0 | 0.14 ± 0.01 | 0.15 ± 0.02 | 1.0 | 1.7 |
| 16:1n-9 | 1.48 ± 0.07 | 1.34 ± 0.06 | 10.3 | 15.2 |
| 16:0 | 4.2 ± 0.2 | 2.8 ± 0.1 | 29.4 | 31.8 |
| 17:1n-10 | – | – | – | – |
| 17:0 | Traces | – | Traces | – |
| 18:2n-6 | – | – | – | – |
| 18:1n-9 | 5.8 ± 0.4 | 2.2 ± 0.1 | 40.6 | 25.0 |
| 18:0 | 0.96 ± 0.05 | 0.70 ± 0.05 | 6.7 | 8.0 |
| 19:0 | – | – | – | – |
| 20:5n-3 | Traces | 0.09 ± 0.01 | Traces | 1.0 |
| 20:4n-3 | Traces | 0.14 ± 0.02 | Traces | 1.6 |
| 20:1n-6 | – | – | – | – |
| 20:0 | – | Traces | – | Traces |
| 22:0 | – | – | – | – |
| 24:0 | – | – | – | – |
| 26:0 | – | – | – | – |
| Sum | 14.3 | 8.8 | – | – |
Cuticular FFA: sum of FFA content in petroleum extract (I), and dichloromethane extract (II)
Data are presented as the means ± standard deviations of three separate analyses performed on different samples
The internal lipids of male L. sericata contained 21 FFA (Table 4). The total free fatty acid content in the internal lipids of the male was 10.1 mg/g of the insect body. The fatty acids occurring in the highest concentrations were 16:1n-9 (14.7%), 16:0 (19.5%) and 18:1n-9 (57.4%). The fatty acids 17:1n-10, 18:2n-6, 19:0, 20:1n-6, 20:0, 22:0, 24:0 and 26:0 occurred only in the internal lipids. On the other hand, 10:0 and 11:0 acids occurred only in the cuticular lipids.
Fatty Acid Composition of Female L. sericata
The cuticular lipids of female L. sericata contained 16 FFA from C6 to C20 (Table 3). Ten FFA from C8 to C18 were identified in the petroleum ether extract (I) and all 16 fatty acids from C6 to C20 in the dichloromethane extract (II). The total free fatty acid content in the cuticle in females was 8.8 μg/g (2.0 μg/g in the petroleum ether extract and 6.8 μg/g in the dichloromethane extract). The percentage contents of fatty acids were very diverse (from traces to 31.2%). The FFA occurring in the highest concentrations were 16:1n-9 (15.2%), 16:0 (31.8%), 18:1n-9 (25.0%) and 18:0 (8.0%).
More fatty acids were present in the internal lipids; they ranged from C6 to C26 (Table 4) (like the internal lipids in the males). The total free fatty acid content in the internal lipids of the female was 7.4 mg/g of the insect body. Twenty four saturated, monounsaturated and polyunsaturated fatty acids were present in the internal lipids. Saturated (16 compounds) and monounsaturated fatty acids (five compounds) were dominant. The FFA occurring in the highest concentrations were 16:1n-9 (17.5%) 16:0 (21.0%) and 18:1n-9 (53.2%). A similar profile of the major compounds was identified in the cuticular lipids, except that the major compound was 18:1n-9 in the internal lipids and 16:0 in the cuticular lipids. The following acids present in the internal lipids were absent from the cuticular lipids: 14:1n-9, 17:1n-10, 17:0, 18:2n-6, 19:0, 20:1n-6, 22:0, 24:0 and 26:0. On the other hand, only 11:0 was absent from the internal lipids of female L. sericata, whereas the cuticular lipids contains traces of it.
Alcohol Composition of Male L. sericata
Table 5 lists the percentage contents of alcohols in the cuticle as well as the alcohol contents calculated per g of insect body. Only five alcohols were found in the cuticular lipids of the males and ranged from C12:0 to C20:0. The compound present in the highest concentrations was C18:0 (0.37 μg/g of the insect body; relative content 55.2% of total alcohols). The total cuticular alcohol content in male L. sericata was only 0.67 μg/g. Only traces of five alcohols were found in the internal lipids. These alcohols have more carbon atoms than the cuticular ones, from C14:0 to C26:0. The internal lipids contain traces of three alcohols (C22:0, C24:0 and C26:0), which were absent from the cuticular lipids. All the identified alcohols were saturated, with even-numbered carbon chains.
Table 5.
Chemical composition of the alcohols found in male of Lucilia sericata
| Content (μg/1 g) | Relative content % (w/w) | |||||
|---|---|---|---|---|---|---|
| Alcohols | Cuticular alcohols (male) | Internal alcohols (male) | Cuticular alcohols (female) | Internal alcohols (female) | Cuticular alcohols (male) | Cuticular alcohols (female) |
| C10 | – | – | Traces | – | – | Traces |
| C12 | 0.05 ± 0.01 | – | 0.11 ± 0.01 | – | 7.5 | 13.8 |
| C14 | 0.13 ± 0.01 | Traces | 0.13 ± 0.01 | Traces | 19.4 | 16.3 |
| C16 | Traces | – | 0.03 ± 0.01 | – | Traces | 3.8 |
| C18 | 0.37 ± 0.03 | – | 0.39 ± 0.03 | – | 55.2 | 48.8 |
| C20 | 0.12 ± 0.01 | Traces | 0.14 ± 0.01 | Traces | 17.9 | 17.5 |
| C22 | – | Traces | Traces | Traces | – | Traces |
| C24 | – | Traces | – | – | – | – |
| C26 | – | Traces | – | – | – | – |
| Sum | 0.67 | Traces | 0.80 | Traces | – | – |
Cuticular alcohols: sum of alcohols content in petroleum extract (I), and dichloromethane extract (II)
Internal alcohols: content of alcohols in extract III
Data are presented as the means ± standard deviations of three separate analyses performed on different samples
Alcohol Composition of Female L. sericata
The cuticular lipids of the female contained seven saturated alcohols with even-numbered carbon chains from C10:0 to C22:0 (Table 5). The alcohol C18:0 was present in the highest concentrations (48.8%) (the same observation as for the male lipids). The total cuticular alcohol content in females of L. sericata was 0.80 μg/g (a little more than in the males). Only three alcohols (C14:0, C20:0 and C22:0) were found in traces in the internal lipids of females.
Cholesterol Content in Male and Female L. sericata
The cholesterol contents in the cuticular lipids of males and females were 7.6 and 11.4 μg/g of the insect body, respectively (Table 6). Considerably more cholesterol was present in the internal lipids of these insects. The quantities of internal cholesterol in female lipids were double those found in males. The cholesterol was identified on the basis of the characteristic ions (as the trimethylsilyl ether).
Table 6.
Chemical composition of the cholesterol found in male and female of Lucilia sericata
| Content (μg/1 g) | ||
|---|---|---|
| Cuticular cholesterol | Internal cholesterol | |
| Male | 7.6 ± 0.4 | 4.9 × 102 ± 0.2 × 102 |
| Female | 11.4 ± 0.8 | 9.7 × 102 ± 0.3 × 102 |
Cuticular cholesterol: sum of cholesterol content in petroleum extract (I), and dichloromethane extract (II)
Internal cholesterol: content of cholesterol in extract III
Data are presented as the means ± standard deviations of three separate analyses performed on different samples
Discussion
Free fatty acids of L. sericata were present not only in the cuticle; large amounts were also detected in the internal lipids. The efficiency of cuticular and internal lipids extraction differed in L. sericata males and females. Amounts of cuticular lipids (mg/g of the insect body) extracted with the use of petroleum ether and dichloromethane from males were 2.1 and 1.1 times higher, respectively, than the amounts of extracts obtained from females. Similarly, amounts of internal lipids extracted from males were 1.3 times higher than analogous extracts obtained from females. The total amounts of extracted lipids (cuticular and internal) comprised 2.99% of male and 2.17% of female wet body weight, respectively. From Gilbert [27] it appears that lipid content in male adults of Calliphora vicina (formerly C. erythrocephala), closely related to L. sericata, and is slightly higher than in females (3.4 vs. 3.3% wet weight). In most insect species the female usually contains more lipids than the male, as a lipid is a most efficient substrate for egg development. However, the reverse may be true for many species and this is especially evident when Lepidoptera are considered [27]. Sexual dimorphism in lipid content in the adult stage was studied in detail in the silk moth Hyalophora cecropia. The tissues of H. cecropia males contain about five times as much lipid per gram of fresh weight as the tissues of the female. The higher concentration of lipids in the male moth is most likely correlated with mating behavior, since the male moth flies relatively great distances in search of virgin females while the female does only limited flying after emergence. The silk moth male appears to utilize lipid as a primary substrate for flight while female H. cecropia converts a large percentage of her endogenous substrate into eggs [27]. In contrast, adults of L. sericata utilize carbohydrates as the main source of flight energy [28]. In spite of intensive studies concerning the flight of L. sericata and some other Diptera species, no sexual difference in the flight behavior and biochemistry was evidenced [29, 30].
Cuticular fatty acids in male and female extracts made up ca 0.14 and 0.12% of all lipids, respectively. The amounts of FFA in cuticular lipids can vary with respect to sex, stage and living conditions. It has been shown that FFA comprise from 2.04 to 0.50% of the lipids in the exuviae of Dendrolimus pini [31] and only trace amounts of FFA were detected in cuticular lipids from nymphs and exuviae of the Silver leaf whitefly, Bemisia argentifolii [32], whereas FFA make up 79.40% of the cuticular lipids of Calliphora vicina larvae [20]. Cuticular FFA in males and females of L. sericata ranged from C6 to C20; moreover, they consisted of both odd- and even-numbered carbon chains. Similar profiles were identified in closely related C. vicina larvae [20]: the fatty acids in this insect ranged from C5 to C20, although the typical cuticular fatty acids ranged from C12 to C20. For example, fatty acids in this range were found in lipids from Frankliniella occidentalis adults and larvae [33], Acanthoscelides obtectus males and females [25], Liposcelis bostrychophila [34] and Fannia canicularis [35]. In our study, the cuticular fatty acids identified in the adult insects were both saturated and unsaturated. Odd- and even-numbered, saturated and unsaturated fatty acids are typically found in many insect species. The presence of polyunsaturated acids 20:4n-3 and 20:5n-3 in cuticular lipids is rather unusual, although these acids were identified in the lipids of the aquatic insect larvae Stictochironomus pictulus [36]. In our work, the females contained these compounds in respective concentrations of 1.6 and 1.0%. Moreover, trace amounts of 20:4n-3 and 20:5n-3 acids were present in the male cuticular lipids. The female extract contained C7 and C20 acids, which were absent from the male extract. On the other hand, C17 acid occurred only in the male extract.
The profiles of predominant cuticular FFA in males and females of L. sericata were similar. The predominant components of males and females consisted of 16:0 and 18:0 and also 16:1n-9 and 18:1n-9 fatty acids. Other cuticular fatty acids were present in much smaller quantities. Only four unsaturated fatty acids were present in both males and females. Internal FFA in males and females of L. sericata made up 40.4 and 37.7% of all lipids, respectively. Free fatty acids were extracted from both sexes of L. sericata with carbon numbers ranging from C6 to C26, so the internal free fatty acid profiles of both adult insects were similar. In this case, the acids present in the highest concentrations in the internal extract were 16:1n-9, 16:0 and 18:1n-9. The contents (%) of 20:5n-3 and 20:4n-3 fatty acids of both sexes were similar; there was a significant difference between the amounts (μg/g) of these acids (male: 0.56 mg/g of the insect body and female: 0.38 mg/g of the insect body). The female extract contained 7:0, and traces of 10:0 and 14:1n-9 acids, which were absent, from the male extract. 22:0, 24:0 and 26:0 acids were present in the internal lipids, which were absent from the cuticular male and female extracts.
The alcohols present in the cuticular lipids of insects usually have an even number of carbons and are saturated [32, 37–39]. In our study, the cuticular lipids of females of L. sericata contained seven even-numbered, saturated alcohols from C10 to C22, but the cuticular lipids of the males contained only five such compounds, from C12 to C20. C10 and C22 alcohols were present only in female cuticular lipids. However, the remaining alcohols were identified in both samples at similar levels. The alcohol present in the highest concentrations in males and females was C18 (55.2 and 48.8%, respectively). The alcohols found in our study ranged from C10 to C24, and similar profiles were identified in Locusta migratoria migratoriodes (from C10 to C34) and Schistocerca gregaria (from C10 to C32) [40]. It is known that the amounts of alcohols in the cuticular lipids of an insect may differ significantly between various species. For example, alcohols made up 42% of all cuticular lipids in pupae of Heliothis virescens [37], but only 3 and 4% in nymphs and exuviae of B. argentifolii [32]. In our work, the cuticular alcohols in male and female L. sericata were <1% of all lipids. Internal lipids of females and males of L. sericata respectively contained three (from C14 to C22) and five (from C14 to C26) alcohols in trace quantities.
Diverse biological functions of cuticular alcohols have been reported. In the European honey bee, alcohols may protect against parasite attack. Extracts of Apis mellifera cocoons containing alcohols of 17–22 carbons induce a strong arrestment response in the mite Varroa jacobsoni [41]. A mixture of short chain alcohols, their acetate derivatives, and (Z)-11-eicosenol secreted by the sting apparatus of the worker honey bee are components of bee alarm pheromones [42]. The sex pheromones of the three most important tortricids of European vineyards, Eupoecilia ambiguella, Sparganothis pilleriana and Lobesia botrana, have been chemically investigated and found to contain up to 15 different straight-chain acetates and alcohols [43]. The role of alcohols found in L. sericata remains unknown.
In cuticular and internal lipids of L. sericata, cholesterol was identified on the basis of the characteristic ions (m/z 129 (100%), 329 (87%), 145 (38%), 121 (36%), 353 (32%) and M+· 458) [44] (as the trimethylsilyl ether). Female lipids contained twice as much cholesterol as male lipids. Internal cholesterol in male and female extracts made up 2 and 5% of all lipids, respectively. The respective quantities of cholesterol obtained from male and female L. sericata were 0.49 ± 0.02 and 0.97 ± 0.03 mg/g of the insect body, respectively. There was a tenfold higher concentration of cholesterol in internal lipids. Sterols are minor constituents of cuticular lipids [45]. The cholesterol content in cuticular male and female lipids was 7.6 ± 0.4 and 11.4 ± 0.8 μg/g, respectively. Assuming that sterols are mandatory for egg production and normal embryonic development, a higher concentration of internal cholesterol in L. sericata females seems justified. In contrast, the physiological role of cuticular cholesterol remains obscure.
Naturally occurring entomopathogenic fungi are important regulatory factors of insect populations. Conidiobolus coronatus, a cosmopolitan soil fungus causing rapid death of susceptible insects, due to the secretion of toxic metabolites [46, 47] was used in current studies. Exposure of L. sericata to sporulating fungal colonies resulted in prompt death of both, males and females while larvae and pupae remained unharmed and developed normally. Similarly, the larvae of closely related C. vicina showed amazing resistance to C. coronatus, while exposure of two lepidopteran larvae, G. mellonella and D. pini, resulted in their death. Microscopic studies revealed that the conidia of C. coronatus did not germinate on the cuticle of C. vicina larvae while the cuticles of both lepidopteran larvae were infiltrated by fungal hyphae [47, 48]. The impressive C. vicina resistance to fungus is accompanied by a high resistance of the cuticle to degradation by fungal proteases and a high antiproteolytic capacity of insect hemolymph. On the other hand, the immune system of challenged larvae shows very low activity of both, cellular and humoral components. It seems that the significant protection provided by the C. vicina cuticle reduces potentially costly defense responses within the hemocoel. Investment in cuticular protection comes at the cost of low phenoloxidase, lysozyme, encapsulation, and phagocytic activities [48]. The cuticular fatty acids profile of C. vicina larvae significantly differs from profiles of D. pini and G. mellonella. Data from in vitro cultures of C. coronatus in media supplemented with various fatty acids showed strong fungistatic effects of 18:2n-6, 18:3n-6, 20:1n-3, and 20:0 [21]. It should be pointed out that all these compounds are missing in the cuticular lipids of L. sericata adults (traces of 20:0 were detected in females only). On the other hand, 16:1n-9 stimulating fungal virulence [21] is present at high concentrations in both males and females (10.3 and 15.2% of all cuticular lipids, respectively) suggesting that this compound may be responsible for the prompt death of flies exposed to C. coronatus colonies. Analysis of cuticular lipids of L. sericata larvae and pupae (currently underway in our laboratories) should provide information on whether the high susceptibility of L. sericata adults to fungal infection opposed to the resistance of the larvae and pupae is linked with diverse cuticular lipid profiles. Work on the effects of new compounds found in adults of L. sericata on the pathogenicity potential of this entomopathogen is in progress. Knowledge of the role of cuticular lipids in fungal interaction with the insect host can be expected to contribute to a better understanding of the nature of fungal pathogenicity.
Acknowledgments
We would like to express our gratitude to Dagmara Gąsiewska for her assistance. Financial support was provided by the Polish Ministry of Science and Higher Education for 2010–2013 grants: N N303 504238 and DS 8110-4-0085-1.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Abbreviations
- SAM
Sabouraud agar medium
- TIC
Total ion current
- SIM
Single ion monitoring
- EI
Electron impact
- BSTFA
N,O-Bis(trimethylsilyl) trifluoroacetamide
- TMCS
Trimethylchlorosilane
- M+·
Molecular ion
- TMSi
Trimethylsilyl derivatives
- FFA
Free fatty acids
- 7-T, 23:1
7-Tricosene
- 7-P, 25:1
7-Pentacosene
- 7,11-HD, 27:2
7,11-Heptacosadiene
- 7,11-ND, 29:2
7,11-Nonacosadiene
References
- 1.Mateos M, Leon A, Gonzalez-Herranz P, Burgos J, Lopez-Mondejar JA, Baquero F. Lucilia sericata infestation of the skin openings for the bone traction device in lengthening of the tibia: apropos of a case [in Spanish] Enferm Infecc Microbiol Clín. 1990;8:365–367. [PubMed] [Google Scholar]
- 2.Morsy TA, Fayad ME, Salama MM, Sabry AH, el-Serougi AO, Abdallah KF. Some myiasis producers in Cairo and Giza abattoirs. J Egypt Soc Parasitol. 1991;21:339–346. [PubMed] [Google Scholar]
- 3.Granz W, Schneider D, Schumann H. Human myiasis in middle Europe [in German] Z gesamte innere Med Grenz. 1975;30:293–301. [PubMed] [Google Scholar]
- 4.Prete PE. Growth effects of Phaenicia sericata larval extracts on fibroblast: mechanism for wound healing by maggot therapy. Life Sci. 1996;60:505–510. doi: 10.1016/S0024-3205(96)00688-1. [DOI] [PubMed] [Google Scholar]
- 5.Daeschlein G, Mumcuoglu KY, Assadian O, Hoffmeister B, Kramer A. In vitro antibacterial activity of Lucilia sericata maggot secretions. Skin Pharmacol Physiol. 2007;20:112–115. doi: 10.1159/000097983. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Wang S, Diao Y, Zhang J, Lv D. Fatty acid extracts from Lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity. Lipids Health Dis. 2010;9:24–32. doi: 10.1186/1476-511X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadley NF, Blomquist GJ, Lanham UN. Cuticular hydrocarbons of four species of Colorado Hymenoptera. Insect Biochem. 1981;11:173–177. doi: 10.1016/0020-1790(81)90093-7. [DOI] [Google Scholar]
- 8.Roberts DR, Hajek AE. Entomopathogenic fungi as bioinsecticides. In: Leatham GF, editor. Frontiers in industrial mycology. New York: Chapman and Hall; 1992. pp. 144–159. [Google Scholar]
- 9.Clarkson JM, Charnley AK. New insight into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 1996;4:197–203. doi: 10.1016/0966-842X(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 10.Khachatourians GG (1996) Biochemistry and molecular biology of entomopathogenic fungi. In: Howard DH, Miller JD (eds) The Mycota VI. Human and animal relationships. Springer, Berlin, pp 331–363
- 11.Shah PA, Pell JK. Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol. 2003;61:413–423. doi: 10.1007/s00253-003-1240-8. [DOI] [PubMed] [Google Scholar]
- 12.Faria M, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43:237–256. doi: 10.1016/j.biocontrol.2007.08.001. [DOI] [Google Scholar]
- 13.Vilcinskas A, Götz P. Parasitic fungi and their interactions with the insect immune system. Adv Parasitol. 1999;43:267–313. doi: 10.1016/S0065-308X(08)60244-4. [DOI] [Google Scholar]
- 14.Gillespie JP, Bailey AM, Cobb B, Vilcinskas A. Fungi as elicitors of insect immune responses. Arch Insect Biochem Physiol. 2000;44:49–68. doi: 10.1002/1520-6327(200006)44:2<49::AID-ARCH1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Fargues J, Delmas JC, Lebrun RA. Fecundity and egg fertility in the adult Colorado beetle (Leptinotarsa decemlineata) surviving larval infection by the fungus Beauveria bassiana. Entomol Exp Appl. 1991;61:45–51. doi: 10.1111/j.1570-7458.1991.tb02394.x. [DOI] [Google Scholar]
- 16.Napolitano R, Juarez MP. Entomopathogenous fungi degrade epicuticular hydrocarbons of Triatoma infestans. Arch Biochem Biophys. 1997;344:208–214. doi: 10.1006/abbi.1997.0163. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Aoki J. Toxicity of free fatty acids on the larval surfaces of two lepidopterous insects towards Beauveria bassiana (Bals.) Vuill. and Poecilomyces fumoso-roseus (Wise) Brown et Smith (Deuteromycetes: Moniliales) Appl Entomol Zool. 1983;18:225–233. [Google Scholar]
- 18.Smith RJ, Grula EA. Nutritional requirements for conidial germination and hyphal growth of Beauveria bassiana. J Invertebr Pathol. 1981;37:222–230. doi: 10.1016/0022-2011(81)90079-3. [DOI] [Google Scholar]
- 19.Kerwin JL. Chemical control of the germination of asexual spores of Entomophthora culicis, a fungus parasitic on dipterans. J Gen Microbiol. 1982;128:2179–2186. [Google Scholar]
- 20.Gołębiowski M, Maliński E, Boguś MI, Kumirska J, Stepnowski P. The cuticular fatty acids of Calliphora vicina, Dendrolimus pini and Galleria mellonella larvae and their role in resistance to fungal infection. Insect Biochem Mol Biol. 2008;38:619–627. doi: 10.1016/j.ibmb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Boguś MI, Czygier M, Gołębiowski M, Kędra E, Kucińska J, Mazgajska J, Samborski J, Wieloch W, Włóka E. Effects of insect cuticular fatty acids on in vitro growth and pathogenicity of the entomopathogenic fungus Conidiobolus coronatus. Exp Parasitol. 2010;125:400–408. doi: 10.1016/j.exppara.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Wieloch W, Boguś MI. Exploring pathogenicity potential of Conidiobolus coronatus against insect larvae in various infection conditions. Pesticides. 2005;4:133–137. [Google Scholar]
- 23.Sehnal F. A critical study of the biome and biometry of the wax moth Galleria mellonella raised in varying conditions. Z Wiss Zool. 1966;174:53–82. [Google Scholar]
- 24.Wieloch W (2006) Toxic metabolites produced by Conidiobolus coronatus. Ph.D. thesis. Institute of Parasitology Polish Academy of Sciences, Warszawa, Poland
- 25.Gołębiowski M, Maliński E, Nawrot J, Stepnowski P. Identification and characterization of surface lipid components of the dried-bean beetle Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) J Stored Prod Res. 2008;44:386–388. doi: 10.1016/j.jspr.2008.02.010. [DOI] [Google Scholar]
- 26.Gołębiowski M, Boguś MI, Paszkiewicz M, Stepnowski P. Cuticular lipids of insects as a potential biofungicides: methods of lipids composition analysis. Anal Bioanal Chem. 2011;399:3177–3191. doi: 10.1007/s00216-010-4439-4. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert LI. Lipid metabolism and function in insects. In: Beament JWL, Treherne JE, Wigglesworth VB, editors. Advances in insect physiology. London: Academic Press; 1967. pp. 69–211. [Google Scholar]
- 28.Mathur CF, Yurkiewicz WJ. Incorporation of glucose-U-14C into lipids of the blowfly during flight. J Insect Physiol. 1969;15:1567–1571. doi: 10.1016/0022-1910(69)90176-0. [DOI] [PubMed] [Google Scholar]
- 29.Yurkiewicz WJ, Smyth T. Effect of temperature on flight speed of the sheep blowfly. J Insect Physiol. 1966;12:189–194. doi: 10.1016/0022-1910(66)90135-1. [DOI] [PubMed] [Google Scholar]
- 30.Dean TJ (2003) Fastest flyer. In: Book of insect records, chap. 1. University of Florida. http://entnemdept.ufl.edu/walker/ufbir/chapters/chapter_01.shtml
- 31.Gołębiowski M, Boguś MI, Paszkiewicz M, Stepnowski P. The composition of the free fatty acids from Dendrolimus pini exuviae. J Insect Physiol. 2010;56:391–397. doi: 10.1016/j.jinsphys.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Buckner JS, Hagen MM, Nelson DR. The composition of the cuticular lipids from nymphs and exuviae of the silver leaf whitefly, Bemisia argentifolii. Comp Biochem Physiol. 1999;124B:201–207. [Google Scholar]
- 33.Gołębiowski M, Maliński E, Nawrot J, Szafranek J, Stepnowski P. Identification of the cuticular lipid composition of the western flower thrips Frankliniella occidentalis. Comp Biochem Physiol. 2007;147B:288–292. doi: 10.1016/j.cbpb.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Howard RW, Lord JC. Cuticular lipids of the booklouse, Liposcelides bostrychophola: hydrocarbons, aldehydes, fatty acids, and fatty acid amides. J Chem Ecol. 2003;29:615–627. doi: 10.1023/A:1022806922246. [DOI] [PubMed] [Google Scholar]
- 35.Kerwin JL. Fatty acid regulation of the germination of Erynia variabilis conidia on adults and puparia of the lesser housefly, Fannia canicularis. Can J Microbiol. 1984;30:158–161. doi: 10.1139/m84-025. [DOI] [Google Scholar]
- 36.Kiyashko SI, Imbs AB, Narita T, Svetashev VI, Wada E. Fatty acid composition of aquatic insect larvae Stictochironomus pictulus (Diptera: Chironomidae): evidence of feeding upon methanotrophic bacteria. Comp Biochem Physiol. 2004;139B:705–711. doi: 10.1016/j.cbpc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Buckner JS, Mardaus MC, Nelson DR. Cuticular lipid composition of Heliothis virescens and Helicoverpa zea pupae. Comp Biochem Physiol. 1996;114B:207–216. [Google Scholar]
- 38.Buckner JS, Nelson DR, Mardaus MC. The lipid composition of the wax particles from adult whiteflies, Bemisia tabaci and Trialeurodes vaporariorum. Insect Biochem Mol Biol. 1994;24:977–987. doi: 10.1016/0965-1748(94)90135-X. [DOI] [Google Scholar]
- 39.Nelson DR, Fatland CL, Buckner JS, Freeman TP. External lipids of adults of the giant whitefly, Aleurodicus dugesii. Comp Biochem Physiol. 1999;123B:137–145. doi: 10.1016/s0305-0491(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 40.Oraha VS, Lockey KH. Cuticular lipids of Locusta migratoria migratoriodes, Schistocerca gregaria and other orthopteran species. Polar components. Comp Biochem Physiol. 1990;95B:603–608. [Google Scholar]
- 41.Donze G, Schnyder-Candrian S, Bogdanov S, Diehl PA, Guerin PM, Kilchenmann V, Monachon F. Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch Insect Biochem Physiol. 1998;37:129–145. doi: 10.1002/(SICI)1520-6327(1998)37:2<129::AID-ARCH2>3.0.CO;2-P. [DOI] [Google Scholar]
- 42.Pickett JA, Williams IH, Martin AP. (Z)-11-eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae) J Chem Ecol. 1982;8:163–175. doi: 10.1007/BF00984013. [DOI] [PubMed] [Google Scholar]
- 43.Arn H, Rauscher S, Guerin P, Buser HR. Sex pheromone blends of three tortricid pests in European vineyards. Agric Ecol Environ. 1998;21:111–117. doi: 10.1016/0167-8809(88)90143-0. [DOI] [Google Scholar]
- 44.Steel G, Henderson W. Rapid method for detection and characterization of steroids. Anal Chem. 1972;44:1302–1304. doi: 10.1021/ac60315a053. [DOI] [PubMed] [Google Scholar]
- 45.Lockey KH. Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol B. 1988;89:595–645. [Google Scholar]
- 46.Boguś MI, Scheller K. Extraction of an insecticidal protein fraction from the pathogenic fungus Conidiobolus coronatus. Acta Parasitol. 2002;47:66–72. [Google Scholar]
- 47.Boguś MI, Kędra E, Bania J, Szczepanik M, Czygier M, Jabłoński P, Pasztaleniec A, Samborski J, Mazgajska J, Polanowski A. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J Insect Physiol. 2007;53:909–922. doi: 10.1016/j.jinsphys.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Boguś MI, Szczepanik M. Histopathology of Conidiobolus coronatus infection in Galleria mellonella larvae. Acta Parasitol. 2000;45:48–54. [Google Scholar]