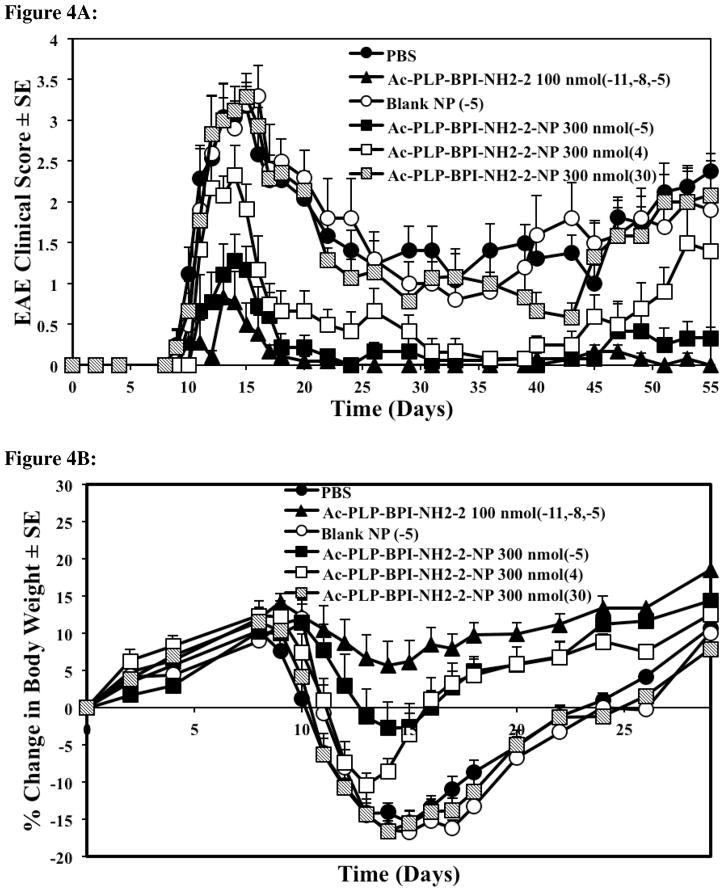
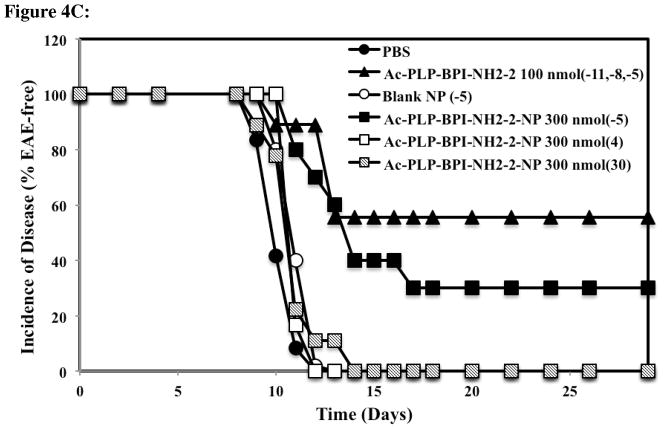
Figure 4.
Comparison of the in vivo activity of Ac-PLP-BPI-NH2-2 in solution, Ac-PLP-BPI-NH2-2-NP, blank colloidal gel, and PBS using clinical disease scores. In the controlled-release treatments, different groups of SJL/J mice received a one-time injection of 300 nmol peptide-gel formulation on days −5, 4, or 30. Mice were also treated with a one-time injection of blank colloidal gel 5 days prior to the induction of disease. The positive control group (Ac-PLP-BPI-NH2-2, 100 nmol/injection) was treated on days −11, −8, and −5. The mice group receiving PBS was also injected on days −11, −8, and −5. (A) The mice treated with Ac-PLP-BPI-NH2-2-NP on day −5 and day 4 had significantly lower clinical scores compared to negative control, PBS, or blank-NP (p < 0.01, through days 12–17, and p < 0.0001, through days 45–55). No significant difference was observed between Ac-PLP-BPI-NH2-2-NP (−5) and the positive control (p > 0.05, through days 45–55). No significant difference in clinical scores was observed when comparing the mice treated with Ac-PLP-BPI-NH2-2-NP on day 30 to negative controls (p > 0.05, through days 45–55). (B) The mice treated with Ac-PLP-BPI-NH2-2-NP on day −5 and day 4 had significantly less changes in body weight compared to PBS, or blank-NP (p < 0.01, through days 12–24). (C) Compared to PBS and the blank colloidal gel control groups, a delay in the onset of disease was apparent in the mice group treated with Ac-PLP-BPI-NH2-2-NP on day −5. The results are expressed as the mean ± S.E. (n ≥ 6).