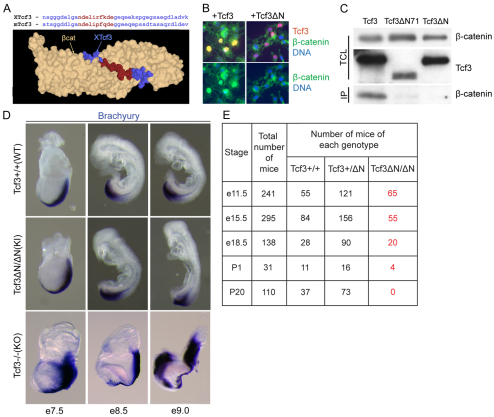
Fig. 1.
Tcf3–β-catenin interaction is required after gastrulation. (A) Three-dimensional structure of the Tcf3–β-catenin interaction (Graham et al., 2000). Amino acid sequence of Tcf3 proteins from Xenopus and mouse are aligned above the structure. Red denotes Tcf3 residues of the core β-catenin interaction domain mutated in Tcf3ΔN. (B) Immunofluorescent staining detects nuclear co-localization in Cos-7 cells of endogenous β-catenin (green) and transiently transfected Tcf3 (red, top panels only). Expression of the Tcf3ΔN mutant protein (+Tcf3ΔN, right panels) failed to cause nuclear β-catenin localization. (C) Co-immunoprecipitation (IP) using a Tcf3-specific antibody and total cell lysate (TCL) from Cos-7 cells transiently transfected with the Tcf3 expression plasmid for wild-type (WT) (Tcf3), a mutant lacking the first 71 residues (Tcf3ΔN71), or the Tcf3ΔN knock-in mutation. (D) Whole-mount in situ hybridization with a labeled brachyury cRNA probe on Tcf3+/+ (WT), Tcf3ΔN/ΔN (knock-in, KI) and Tcf3–/– (knockout, KO) embryos. Expression patterns and morphology of KI embryos resembled those of WT embryos, and KI embryos did not display the defects observed in KO embryos. (E) Recovery of embryos of the indicated genotypes obtained from timed pregnancies of Tcf3+/ΔN × Tcf3+/ΔN matings.