Figure 1.
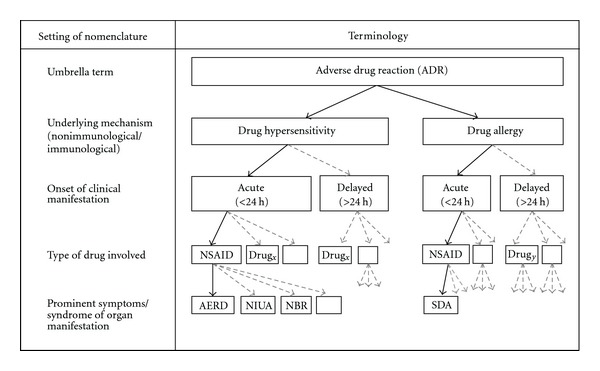
Allocation of terms used for adverse reactions to drugs. The diagram files the term AERD in the context of ADR, drug hypersensitivity, and drug allergy. The terms were gathered from “Report of the Nomenclature Review Committee of the World Allergy Organization” [7], and the proposed classification of allergic and pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes [9]; AERD: aspirin-exacerbated respiratory diseases, NSAID: nonsteroidal anti-inflammatory drugs, NIUA: NSAID-induced urticaria/angioedema, NBR: NSAID-blended reaction, SDA: single drug-induced anaphylaxis. Definition of ADR according to the World Health Organization [10]: any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy. This definition excludes therapeutic failures, intentional and accidental poisoning (i.e., overdose), and drug abuse.
