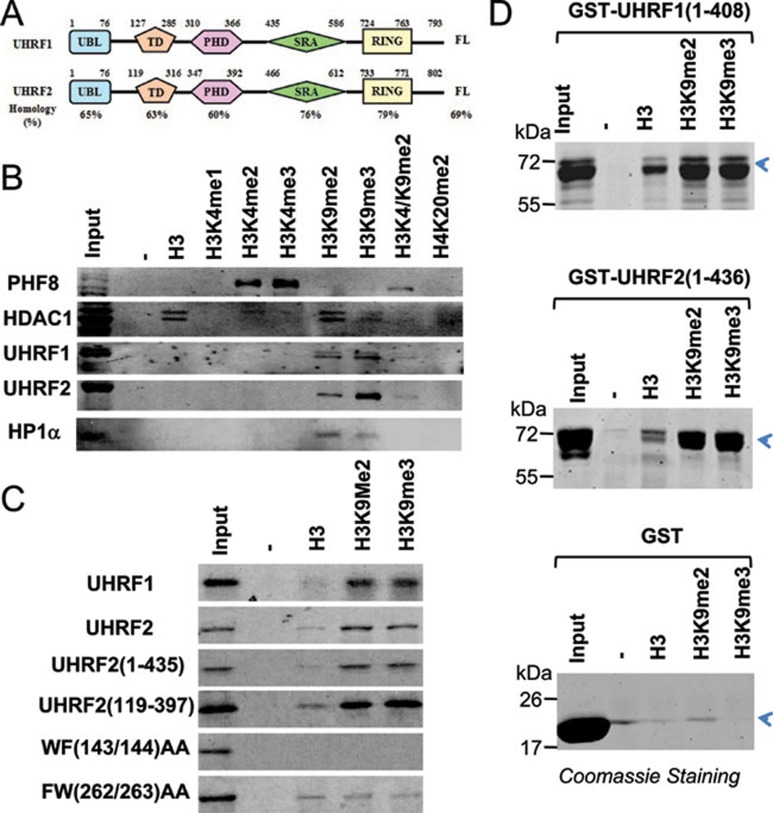
Figure 1.
UHRF2 recognizes specifically H3K9 methylation by its tandem tudor domain. (A) A diagram illustrating the structure and sequence similarity between human UHRF1 and UHRF2. UBL, ubiquitin-like domain; TD, tandem tudor domain; PHD, plant homeodomain; SRA, SET and ring associated domain; Ring, really interesting new gene domain. (B) Binding of UHRF2 to a panel of methylated histone H3 and H4 peptides in comparison to other known histone-binding proteins. Pull-downs with HeLa nuclear extracts and various histone peptides were performed and the binding of UHRF1, UHRF2, HP1α, PHF8 and HDAC1 were revealed by western blot analysis. (C) The tandem tudor domain determines the H3K9me2 binding specificity. In vitro synthesized, 35S-met-labeled UHRF2 and deletion or point mutation mutants were subjected to pull-down assays with H3 and H3K9me2 and H3K9me3 peptides. The binding of various UHRF2 proteins were revealed by autoradiography. (D) Recombinant UHRF1 and UHRF2 bound H3K9me2/3 peptides in vitro. Purified GST-UHRF1 (aa 1-436) and GST-UHRF2 (aa 1-408) were subjected to pull down assays and the proteins bound to the peptides were resolved by SDS-PAGE and revealed by Coomassie blue staining.